Photo-coupled electrocatalytic oxygen reduction to hydrogen peroxide using metal-free CNT-threaded oxidized g-C3N4
Abstract
Hydrogen peroxide (H2O2) has been widely used in environmental cleaning, hospital disinfecting and chemical engineering. Compared to the traditional anthraquinone oxidation method, the electrocatalytic two-electron oxygen reduction reaction (2e-ORR) to produce H2O2 has become a promising alternative due to its green, safety and reliability. However, its industrial application is still limited by the slow reaction kinetics and low selectivity due to the competitive reaction of the 4e-ORR to H2O. Herein, we prepare a novel photoresponsive metal-free electrocatalyst based on oxidized g-C3N4/carbon nanotubes (OCN/CNTs) and introduce an external light field to realize the high-performance electrocatalytic 2e-ORR to produce H2O2. Impressively, the OCN/CNT electrocatalyst exhibits an outstanding H2O2 productivity of 30.7 mmol/gcat/h with a high faradaic H2O2 efficiency of 95%. The oxygen-containing groups of the OCN/CNTs promote the adsorption of oxygen intermediates and the photo-coupled electrocatalysis simultaneously improves the electron transport efficiency and enhances the electrocatalytic selectivity.
Keywords
INTRODUCTION
Hydrogen peroxide (H2O2) is a multifunctional strong oxidant that has been widely used in major industrial production, including chemical synthesis[1,2], sterilization disinfection[3,4] and environmental governance[5,6]. H2O2 is also regarded as a new clean fuel energy due to its extremely high transportation safety[7-10]. The traditional method of anthraquinone oxidation to produce H2O2 requires high energy consumption and also causes serious pollution[11,12]. Recently, the electrocatalytic production of H2O2 through the two-electron oxygen reduction reaction (2e-ORR) has arisen significant attention due to its safety, portability and environmental friendliness, which can effectively avoid the large-scale transportation of H2O2 and realize its in-time production and use[13-15]. High-efficiency, low-cost electrocatalysts with excellent selectivity are pivotal for a high-performance electrocatalytic 2e-ORR to produce H2O2. Although traditional noble metal-based catalysts, including Pt, Au and their alloys, exhibit good electrocatalytic activity and 2e-ORR selectivity, their scarcity and high cost and the complexity of electrode forming strongly impede their practical applications[16,17]. Therefore, transitional metal-based and metal-free catalysts are constantly being designed and developed toward the electrocatalytic 2e-ORR and are close to the electrocatalytic performance of noble metal-based catalysts[18-21].
Due to their high conductivity and structural tunability as metal-free catalysts, carbon-based 2e-ORR catalysts, including carbon nanotube (CNTs), graphene and carbon nanofibers, are regarded as the most promising potential alternatives to the abovementioned noble metal-based catalysts[22-25]. However, for pure carbon-based catalysts, their poor intrinsic catalytic activity cannot satisfy the demand for H2O2 production. Furthermore, although the electrocatalytic activity and selectivity of carbon-based catalysts can be significantly improved by constructing structural defects[24,26] and doping with heteroatoms, such as N, B and O[22,27,28], their catalytic performance is still relatively moderate with significant potential for improvement.
It is noteworthy that in addition to the modification of electrocatalysts, the introduction of external fields, including light, thermal, magnetic and piezoelectric fields, could further improve the catalytic performance[29-32]. Innovatively, Shen et al. found that Co3O4 nanotube array-supported copper nanoparticles (NPs) showed a higher CO2 conversion rate under illumination and demonstrated that the light field helped to reduce the potential barrier of electrocatalysis and promoted the electrode kinetics[33]. In general, when external light fields are introduced into traditional electrocatalysis, the specific electronic properties of the electrocatalyst, such as electron transfer, band bending, Fermi energy levels and intermediate desorption energy, are altered, which significantly changes the intrinsic path and performance of the catalysts[34-36]. More importantly, under the synergistic effect of photocatalysis and electrocatalysis, the use of a photo-coupled electrocatalytic system to enhance the 2e-ORR performance could be a promising strategy for H2O2 production.
As a metal-free conjugated polymer, graphitic carbon nitride (g-C3N4) NPs have attracted significant attention in the application of the electrocatalytic ORR because of their unique three-dimensional morphology, tunable electronic structure, superior chemical stability, abundant pyridine nitrogen content, high durability and low cost[37-40]. More importantly, g-C3N4 possesses a good adsorption capacity for oxygen due to its rich nitrogen content and has been successfully applied in the photocatalytic 2e-ORR to produce H2O2[41-43]. Nevertheless, the poor H2O2 selectivity and high electrical resistance result in inferior electrocatalytic H2O2 production of the pristine g-C3N4. Various studies have shown that introducing a co-catalyst and constructing a heterojunction system can significantly improve the charge migration and ORR performance of g-C3N4[44-46].
Herein, we propose a light-field enhancement route for improving the electrocatalytic 2e-ORR to produce H2O2 by designing a novel photocoupled electrocatalyst based on oxidized g-C3N4/CNTs. Compared to standard electrocatalysis, photo-coupled electrocatalysis can reduce the electrode overpotentials, improve reaction kinetics and promote the electrode stability to facilitate catalytic performance. The CNTs can greatly promote the charge separation and light absorption of g-C3N4 under the introduction of illumination, exposing more electrons for O2 reduction. Furthermore, the g-C3N4/CNT hybrid is further oxidized to add more oxygen-containing groups. Rotating disk electrode (RDE) measurements and density functional theory (DFT) calculations illustrate that the combination of OCN and CNTs effectively improve the H2O2 output and enhance the 2e-ORR selectivity.
EXPERIMENTAL METHODS
Preparation of OCN/CNTs
In a typical synthesis process, the GCN was obtained by 15 g of melamine calcined in a muffle furnace at 500 °C for 4 h at a heating rate of 2.19 °C/min. The CNT-threaded GCN-NPs were prepared by nitric acid oxidation. Firstly, the GCN nanorods (GCN-NRs) were synthesized by a hydrothermal treatment[47]. Specifically, 1.5 g of GCN and 1.5 g of urea were dissolved in 150 mL of deionized water and sonicated for 3 h until the solid matter was completely dispersed. Afterward, the solution mixture was transferred into a Teflon-stainless autoclave, followed by the synthetic reactions in an oven at 170 °C for 24 h. Subsequently, a certain amount of GCN-NRs (x = 100, 200, 300 or 400 mg) and 1 mg of CNTs were dissolved in 200 mL of 12 M nitric acid in a three-neck round-bottom flask to synthesize the OCN-x/CNTs (x = 100, 200, 300 or 400 mg)[22]. The effect of different acidification times of the sample was subsequently investigated on the OCN-200/CNTs-y (y = 0, 6, 12, 18 or 24 h). The OCN/CNTs were collected by centrifuging in deionized water and ethanol several times until the pH of the solution reached neutral and dried overnight in a vacuum at 60 °C. The complementary information regarding the electrode preparation, characterization and photoelectric performance measurements of the electrodes, the detection of H2O2 concentration, the RDE measurements and DFT calculation methods are given in the Supporting Information.
RESULTS AND DISCUSSION
Preparation of OCN/CNT catalysts
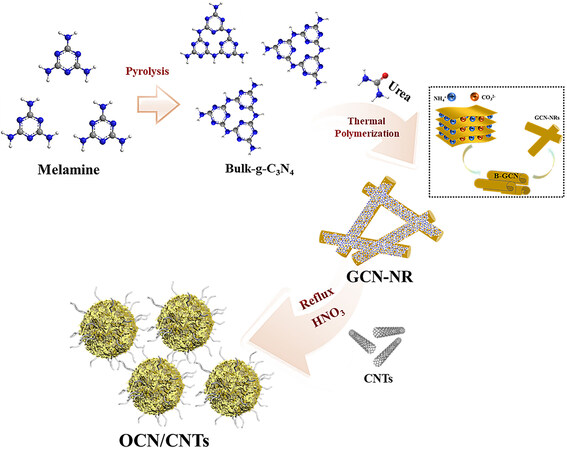
As shown in Figure 1, the metal-free OCN/CNTs catalyst was synthesized via a three-step approach. In the first step, the bulk g-C3N4 (B-GCN) was obtained by the high-temperature pyrolysis of melamine and was mainly composed of flake accumulation [Supplementary Figure 1A and B]. Next, through thermal polymerization, the B-GCN was rolled up and then formed into the GCN-NRs under the interaction between NH4+ and CO32- originating from urea[47]. Finally, the oxidized carbon nitride OCN/CNTs catalysts were prepared by nitric acid oxidation using the reflux condensation method[41]. It is noteworthy that the mass of the CNTs was fixed at 1.0 mg, the samples with different weights of g-C3N4 were denoted as OCN-x/CNTs (x = 100, 200, 300 or 400 mg) and the samples with different oxidation times were labeled as OCN-200/CNTs-y (y = 0, 6, 12, 18 or 24 h). In the oxidation process, the GCN-NRs [Supplementary Figure 1C and D] were transformed into smaller-sized oxidized carbon nitride nanoparticles (OCN-NPs)
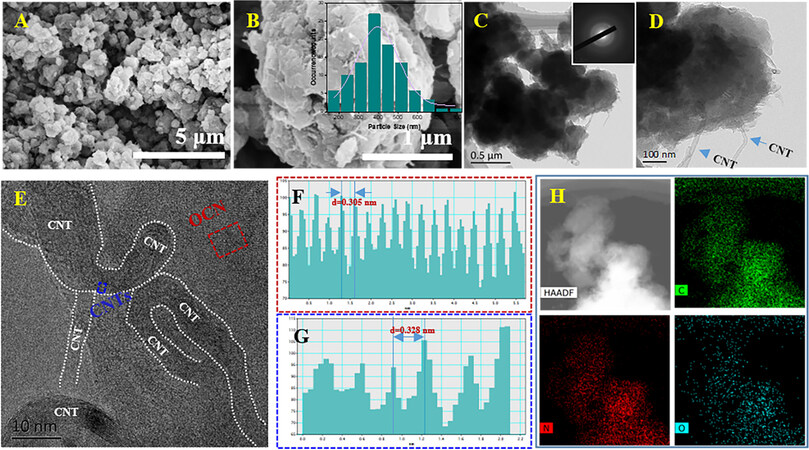
Figure 2. A-B: SEM images of OCN/CNTs (inset: corresponding size distribution histogram of OCN-NPs). C-D: TEM images of OCN/CNTs (inset: SAED image for OCN/CNTs). E: HRTEM image of OCN/CNTs. F-G: Lattice spacing for g-C3N4 and CNTs. H: HAADF image of OCN/CNTs and corresponding EDS mapping images for C, N and O elements.
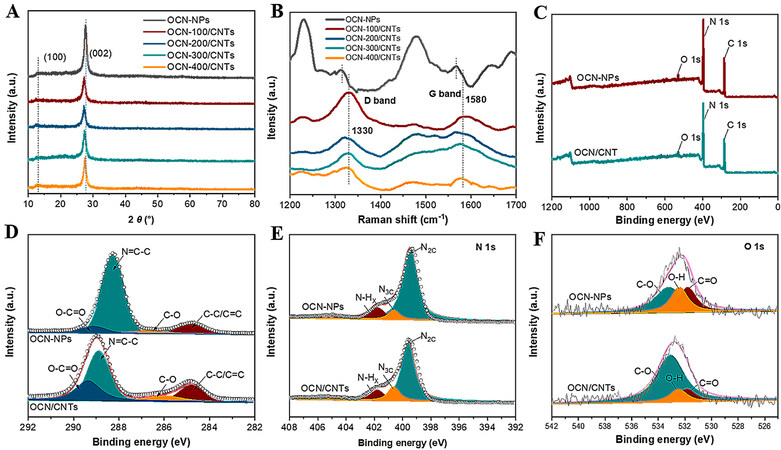
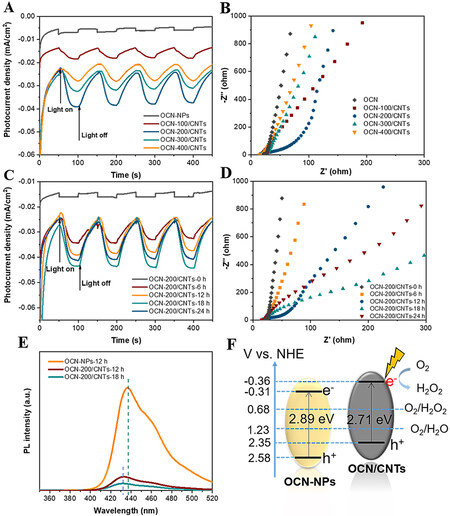
Figure 3A shows the X-ray diffraction (XRD) patterns of the OCN-NPs and OCN/CNTs hybrids with different weight ratios of OCN to CNTs. There were two characteristic diffraction peaks at 2θ of 13.1° and 27.6°, which were attributed to the (100) and (002) crystal planes of graphitic carbon nitride, respectively[42,43]. In the XRD patterns of OCN/CNTs hybrids, the (002) peak was slightly shifted accompanied with a decrease in intensity, because the CNTs were inserted between the layers of g-C3N4 and the interlayers were loosened[48]. As the oxidation time increased, the intensity of the (002) diffraction peak decreased with the reduction of layer stacking [Supplementary Figure 2A]. The Raman spectra further confirmed the successful introduction of CNTs, with Figure 3B manifesting the D and G bands of g-C3N4 located at 1316 and 1567 cm-1, respectively. As for OCN/CNTs, the D- and G-band peaks showed obvious spikes and migrated to 1330 and 1580 cm-1 due to the interaction of g-C3N4 and CNTs[27,49]. Furthermore, the G and D bands of the OCN-200/CNTs-y catalysts were not affected under the acidification, as indicated in Supplementary Figure 2B. In the FTIR spectra of the OCN/CNTs, the vibration peaks of ~3430 and ~800 cm-1 were indexed to the -OH and triazine units of the OCN/CNTs, respectively[50]. It is noteworthy that the intensity of the peak at ~3400–3500 cm-1 increased with increasing acidification time, indicating that the increase in acidification time improved the degree of oxidation [Supplementary Figure 3].
Figure 3. A: XRD patterns for all catalysts. B: Raman spectra for all catalysts. C: XPS survey spectra for OCN-NPs and OCN/CNTs. D-F: High-resolution XPS spectra of C 1s, N 1s and O 1s for OCN-NPs and OCN/CNTs.
X-ray photoelectron spectroscopy (XPS) was further conducted to characterize the composition of the OCN-200/CNTs-18h. From the full-scan XPS spectra, the C, N and O elements existed in the OCN-NPs and OCN-200/CNTs-18h catalysts [Figure 3C]. The C 1s spectrum of the OCN-NPs is shown in Figure 3D. There were four peaks at 288.2, 285.87, 289.39 and 284.8 eV for the OCN-NPs, which were ascribed to the bonding of N=C-N, C-O, O-C=O and C-C/C=C, respectively[42,51,52]. After hybridizing with CNTs, the XPS peak of the N=C-N bond shifted to 288.9 eV, implying the π-π conjugation between the CNTs and OCN-NPs. Interestingly, there were obvious increases in the intensity of the peaks for the C-O and O-C=O, indicating that the introduction of CNTs could enrich the oxygen-containing groups. The N 1s spectrum is shown in Figure 3E, with the peaks at 399.59, 400.62 and 401.78 eV attributed to N2C, N3C and N-HX, respectively[43,44]. Similarly, from the O 1s XPS spectra [Figure 3F], the peak areas of C-O, C=O and O-H at 533.1, 532.5, and 531.8 eV became larger after incorporation with CNTs[48,50]. Supplementary Figure 4 and Supplementary Table 1 demonstrated that the increase in the acidification times could enhance the oxygen content of OCN/CNTs.
Photo-coupled electrocatalytic oxygen reduction to H2O2
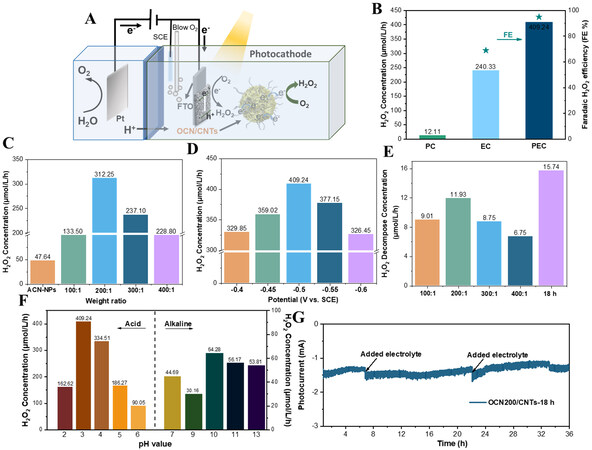
The photo-coupled electrocatalytic ORR installation diagram without any sacrificial agent is shown in Figure 4A. From the UV-vis spectra [Supplementary Figure 5], it could be seen that the GCN could be regarded as an excellent photon absorber under light irradiation and the introduction of the CNTs enhanced the visible-light absorption. Thence, the photo-coupled electrocatalytic 2e-ORR performance of the different catalysts was estimated under visible-light illumination (λ ≥ 400 nm). From Figure 4B, after applying the light field, the yield of H2O2 and the faradaic H2O2 efficiency were significantly improved compared to that of electrocatalysis. Obviously, photo-coupled electrocatalysis is an effective H2O2 production strategy for oxygen reduction. Owing to the excellent photoelectrochemical property, the optimum yield of H2O2 for the OCN/CNTs was 30.7 mmol/gcat/h after normalization, which was ~14 and ~9 times higher than those of pristine g-C3N4 and OCN-NPs, respectively.
Figure 4. A: Schematic of the device for the photo-coupled electrocatalytic synthesis of H2O2. B: H2O2 production of photocatalysis, electrocatalysis and photo-coupled electrocatalytic systems. C: H2O2 production of OCN-x/CNTs (x = 100, 200, 300 or 400). D: Effect of bias voltage on H2O2 production. E: Decomposition capacity of H2O2 in different catalyst samples. F: Effect of different pH on H2O2 production; G: Long-time photocurrent of H2O2 production of the electrode. All the H2O2 production experiments were under the conditions of pH = 3, V = -0.5 V vs. SCE and λ ≥ 400 nm.
The H2O2 output of the catalysts is illustrated in Figure 4C, which demonstrates that the H2O2 evolution rate has been significantly improved after incorporation with CNTs. The H2O2 concentration for the OCN-400/CNTs was nearly four times higher than that of the OCN-NPs. The yield of H2O2 also increased with increasing CNTs content of the OCN-100/CNTs. When the weight ratio of OCN-NPs to CNTs was 200:1, the H2O2 concentration achieved the maximum. However, as the weight ratio of OCN-NPs to CNTs reached 100:1, the photo-induced electron used for the ORR would decrease due to the decrease in the relative content of OCN-NPs, resulting in a decrease in H2O2 production. Subsequently, the effect of acidification time on the yield of H2O2 with OCN/CNTs was also investigated in Supplementary Figure 6. The OCN-200/CNTs-18h with 18h of acidification time showed the best ORR activity for H2O2 generation. This was mainly because the suitable amount of oxygen functional groups could improve the selectivity of H2O2, while too much oxygen content would reduce the conductivity of the catalyst, which was not conducive to producing H2O2[53]. Simultaneously, the yields of H2O2 by using different catalyst samples were also affected by their different photoelectrochemical properties, which was confirmed in the subsequent photoelectrochemical measurements. Compared with the yield of H2O2 of the pure OCN-NPs and OCNTs catalysts, the hybridization of OCN-NPs and CNTs greatly enhanced the efficiency of 2e-ORR to H2O2
The activity of the OCN/CNTs was superior to the OCN-bulk/CNTs and the mixture of OCN/CNTs, demonstrating that the π-π conjugation between the OCN-NPs and CNTs in the OCN/CNTs greatly improved the efficiency of the separation and transmission of photogenerated carriers. Generally, the applied potential could affect the stability of the electrode and there would be a competing HER and 4e-ORR [Equation (2)] in the applied potential[54,55]. Figure 4D shows that the optimal reaction voltage was -0.5 V vs. SCE, indicating that H2O2 could be synthesized efficiently at low bias. Nevertheless, the inferior H2O2 production [Supplementary Figure 9A] and the current [Supplementary Figure 9B] in the electrocatalytic system (-0.7 vs. SCE) were sufficient to reflect that the illumination could provide photogenerated electrons to promote the ORR to synthesize H2O2 for the electrode and the advantages of high efficiency and energy saving in this system.
O2 + 2e- +2H+ → H2O2 (E0 = 0.68 V vs. NHE) (1)
O2 + 4e- +1/2H+ → H2O (E0 = 1.23 V vs. NHE) (2)
2H+ + 2e- → H2 (E0 = 0 V vs. NHE) (3)
O2 + H2O + 2e- → HO2- + HO- (E0 = 0.68 V vs. NHE) (4)
The decomposition rate of H2O2 in the reaction process also played a very important role in the production of H2O2. The decomposition experiments of H2O2 with different electrodes are presented in Figure 4E. Compared to the high yield of H2O2, these decomposition amounts were negligible. It could be seen from Equation (2) that the pH value could greatly affect the performance of the ORR for producing H2O2. Figure 4F demonstrates that the OCN-200/CNTs-18h had extremely high photoelectrochemical activity at pH = 3 due to the high proton concentration. However, when the pH became acidic, the H2O2 yield would be reduced mainly due to the competition of the HER from Equation (3)[48]. Furthermore, the yield of H2O2 decreased as the pH increased from 3 to 7; however, it again increased at pH > 7, giving a peak at pH 10, before falling at a higher pH value. Surprisingly, the electrode achieved H2O2 generation under alkaline conditions, which was mainly due to the peroxygen species produced by Equation (4) at high pH values[55]. The efficient generation of high-value clean energy (H2O2) in a wide range of pH values could provide more possibilities for practical applications.
Supplementary Table 2 illustrates a comparison of the H2O2 yield with current studies. The photocatalytic ORR to H2O2 required the consumption of sacrificial agents and its activity was inferior. Compared to the electrocatalytic ORR[56,57], the photo-coupled electrocatalytic system is relatively low in energy consumption and inexpensive. Our activity was higher than that of photocatalytic technology. For the electrode, the stability of the catalyst during the reaction process was extremely important[40,55]. In the follow-up experiment, we conducted a long-term reaction current test on the OCN-200/CNTs-18h electrode and found that the current remained unchanged after 36 h under the re-addition electrolyte [Figure 4G]. The faradaic H2O2 efficiencies of OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h were 45.96%, 85.83% and 95.00%, respectively [Supplementary Figure 10A], indicating that the OCN-200/CNTs-18h was superior to the ORR to H2O2. From the evaluation of the cycle stability [Supplementary Figure 10B], the output of H2O2 and the FE nearly remained the same in each cycle. The XRD and SEM analysis for the electrode before and after the reaction, also further demonstrated its excellent stability. XRD pattern analysis was carried out on the electrode after the reaction three times. Compared with pure FTO, the [002] crystal peak of g-C3N4 appeared at 27.6° and the peak intensity remained unchanged compared with that before the reaction [Supplementary Figure 11A]. Moreover, the morphology of the catalyst did not change during the SEM analysis [Supplementary Figure 11B]. These results demonstrated that the designed OCN/CNTs electrode in this work had high activity and excellent stability.
Photoelectrochemical properties of OCN/CNTs catalysts
The high charge separation efficiency and longer electron lifetime are beneficial to the improvement of the photoelectrochemical performance. For further understanding the mechanism for the high activity and stability of OCN/CNTs under illumination, the migration, transfer and recombination processes of the photogenerated electron-hole pairs in the OCN/CNTs electrodes were investigated. As shown in Figure 5A, the photocurrent densities for the OCN/CNTs with different ratios of OCN to CNTs under visible-light irradiation were much higher than that of OCN-NPs, demonstrating that the introduction of CNTs improves the charge migration significantly. More importantly, the OCN-200/CNTs show the highest photocurrent response with excellent carrier separation efficiency. In fact, a few OCN-NPs could reduce the quantity of photo-excited electrons, while redundant OCN-NPs would make electrons and holes easy to recombine[58]. The appropriate content of CNTs could effectively improve the conductivity of the OCN/CNTs and facilitate the transport of photogenerated carriers. From the electrochemical impedance spectroscopy (EIS) [Figure 5B], the OCN-200/CNTs possessed the smallest arc radius under low-frequency conditions, indicating the faster charge transfer process at the interface between the catalyst and the electrolyte. Similarly, the higher photocurrent signal [Figure 5C] and smaller arcs [Figure 5D] of OCN-200/CNTs-18h further provide powerful evidence that a suitable content of oxygen-containing groups could promote the separation of photo-induced electrons and separation in OCN/CNTs and then transfer faster to the electrolyte interface for the 2e-ORR to H2O2[3].
Figure 5. A: Photocurrent density of OCN-NPs and CNTs with different weight ratios (λ ≥ 400 nm, -0.5 V vs. SCE). B: EIS Nyquist plots of OCN-NPs and CNTs with different weight ratios. C: Photocurrent density of OCN/CNTs with different acidification times (λ ≥ 400 nm, -0.5 V vs. SCE). D: EIS Nyquist plots of OCN/CNTs with different acidification times. E: PL spectra of OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h. F: Band structure alignments of OCN-NPs and OCN-200/CNTs-18h.
In addition, photoluminescence (PL) spectra were employed to investigate the carrier separation processes of the photogenerated electron-hole pairs in the OCN/CNTs electrode. As shown in Figure 5E, the main emission wavelength of OCN-NPs was located at 437 nm upon excitation at 325 nm. The emission wavelength of OCN/CNTs was 432 nm, slightly blue-shifted by 5 nm. The OCN-200/CNTs-18h showed the lowest PL intensity in comparison with those of the OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h, demonstrating the excellent efficient charge separation and fewer capture centers for the OCN-200/CNTs-18h. The Mott–Schottky plots [Supplementary Figure 12] were used to evaluate the flat band potential. The flat band potentials of the OCN-NPs and OCN-200/CNTs-18h were -0.40 and -0.35 eV, respectively. Generally, the conduction band position is 0.1 eV more negative than the flat band potential[5]. To illustrate the mechanism toward the ORR to H2O2, the electron band structures of the OCN-NPS and OCN-200/CNTs-18h are manifested in Figure 5F. It could be seen that the CB of the OCN-200/CNTs-18h (-0.36 eV) was more negative than that of the OCN-NPs (-0.31 eV), indicating the OCN-200/CNTs-18h was suitable for the 2e-ORR to H2O2. The bandgaps of the OCN-NPs and OCN-200/CNTs-18h were determined by the Kubelka-Munk function, corresponding to 2.89 and 2.71 eV, respectively [Supplementary Figure 13].
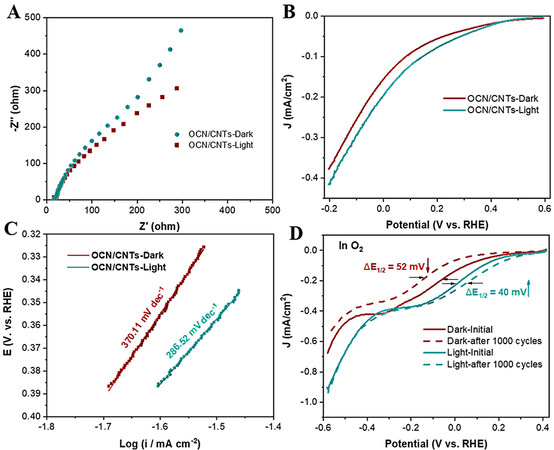
The relevant photoelectrochemical characterization under illumination and dark conditions was further used to understand the attribution of solar energy for the photo-coupled electrocatalytic performance. The smaller arcs and more negative current density of OCN-200/CNTs-18h in the EIS and LSV measurements
Figure 6. A: EIS and B: LSV measurements at 50 mV/s under light and dark for OCN-200/CNTs-18h. C: Corresponding Tafel plots. D: ORR polarization curves at 100 mV/s of OCN-200/CNTs-18h under light and dark before and after 1000 cycles in O2-saturated 0.5 M NaSO4.
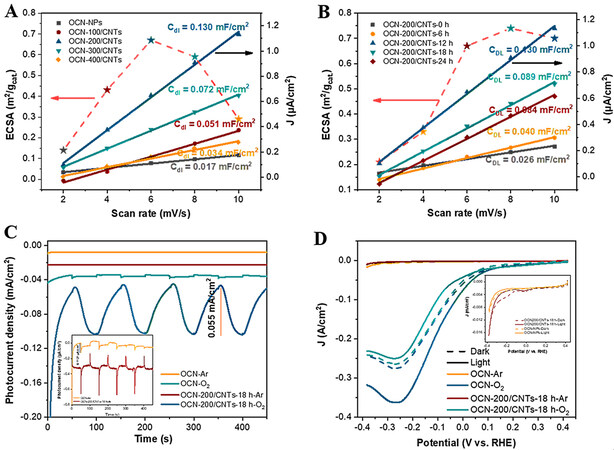
For the photo-coupled electrocatalytic system, the electrochemical activity surface area (ECSA) is an important indicator of catalytic performance since it could directly provide the surface area involved in the electrochemical reaction[59]. The double-layer capacitance (Cdl) was measured from the cyclic voltammetry (CV) curves at different scan rates [Supplementary Figures 17 and 18]. The ECSA of the catalyst samples was calculated by ECSA = Cdl/0.0155 mF cm-2. After normalization, the ECSAs for the OCN-NPs, OCN-100/CNTs, OCN-200/CNTs, OCN-300/CNTs and OCN-400/CNTs were 0.14, 0.43, 0.67, 0.59 and
Figure 7. A-B: Cdl value calculated from CV curves and ECSA (m2/gcat) for OCN-x/CNTs and OCN-200/CNTs-y. C: Photocurrent density of OCN-NPs and OCN/CNTs measured under a saturated Ar (inset) or O2 atmosphere and visible-light illumination (λ ≥ 400 nm, V = -0.5 V vs. SCE); D: LSV of OCN-NPs and OCN/CNTs measured under a saturated Ar (inset) or O2 atmosphere with visible-light illumination (λ ≥ 400 nm, pH = 3).
As shown in Figure 7C, whether in the Ar (inset) or O2 atmosphere, the photocurrent density of the OCN-200/CNTs-18h was always higher than that of the OCN-NPs, indicating the excellent carrier separation efficiency of the OCN-200/CNTs-18h catalyst. Interestingly, under the optimal reaction potential conditions (-0.5 V vs. SCE), the photocurrent response and current value of OCN-200/CNTs-18h were significantly enhanced in an O2 atmosphere compared to in the Ar and air atmospheres [Figure 5C]. The linear sweep voltammetry (LSV) curves of the OCN-NPs and OCN-200/CNTs-18h under saturated Ar or O2 atmospheres also confirmed the above-mentioned phenomenon [Figure 7D]. The OCN-200/CNTs-18h showed a more negative photocurrent under light and oxygen atmospheres than under dark and Ar atmospheres (inset). It is noteworthy that after illumination the current value of OCN-NPs and OCN/CNTs was lower than that under dark conditions and the Ar atmosphere. The same phenomenon occurred in the O2 atmosphere of OCN-NPs, which was mainly caused by the increase in resistance after illumination[55]. This phenomenon highlighted that the OCN-200/CNTs-18h electrode was more conducive to electron transfer for the ORRs under the conditions of illumination and O2 atmosphere. Both the photocurrent and LSV measurements in the Ar and O2 atmospheres demonstrated that the electrode developed in this work with a superior selectivity for the ORR and could greatly inhibit the HER. Furthermore, the H2O2 production under Ar and O2 atmosphere is shown in Supplementary Figure 20, which demonstrates that the synthesized H2O2 was derived from O2.
Mechanism for 2e-ORR on OCN/CNTs
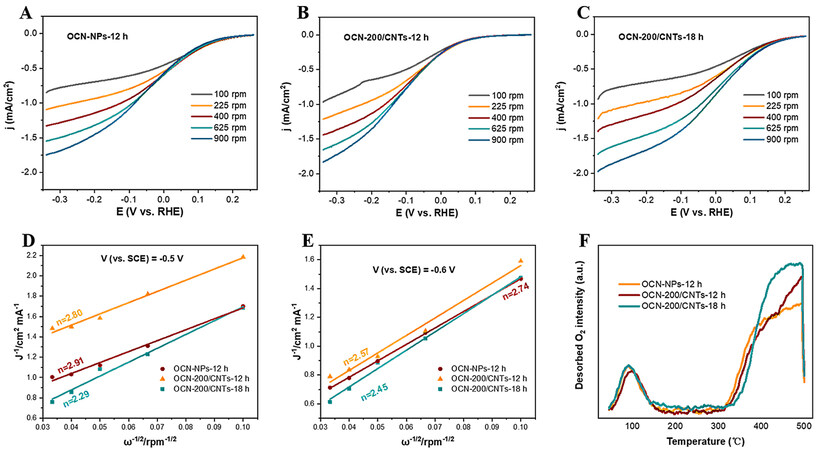
To further study the mechanism of the 2e-ORR on the OCN/CNT-based electrodes, the selectivity of the 2e-ORR was estimated by RDE measurements in 0.05 M Na2SO4 electrolyte under an O2 atmosphere. Based on the analysis for the effect of pH value on the H2O2 production [Figure 3F], the pH value of electrolyte was adjusted to 3 for the RDE measurements with 1 M H2SO4. From the LSV curves of the catalysts under different rotation speeds [Figure 8A-C], the current density of the OCN-200/CNTs-18h was obviously superior to those of the OCN-NPs and OCN-200/CNTs-12h, suggesting that introducing CNTs and oxygen-containing groups improve the 2e-ORR electrocatalytic activity. The numbers of transferred electrons (n) toward the ORR on the catalysts were determined by the Koutecky–Levich (K-L) equation [Equations (5) and (6)]:
Figure 8. A-C: LSVs of OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h catalysts measured on a RDE at different rotating speeds with pH = 3 (scan rate 10 mV/s). D-E: Koutecky–Levich curves of OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h at -0.5 and -0.6 V vs. SCE. F: Comparison of TPD-O2 among OCN-NPs, OCN-200/CNTs-12h and OCN-200/CNTs-18h.
j-1 = jk-1 + b-1ω-1/2 (5)
B = 0.2nFv-1/6 CD2/3 = A*n (6)
where j is the measured current density, jk is the kinetic current density, ω is the rotating speed (rpm), F is the Faraday constant, ν is the kinetic viscosity of water, C is the bulk concentration of O2 in water and D is the diffusion coefficient of O2, respectively.
The contribution of all parameters (denoted as A = 0.032 mC S-1/2) to Equation (6) were determined in a solution of Na2SO4[6]. The slope of the curve (B-1) calculated by linear regression for j-1 and ω-1/2 at a designated voltage could determine the average number of electrons (n) in the ORR. Figure 8D indicates that the measured n value of the OCN-NPs was 2.91, while the n values of OCN-200/CNTs-12h and OCN-200/CNTs-18h were 2.80 and 2.29 at the optimal reaction potential (-0.5 V vs. SCE), respectively. Similarly, the nOCN-NPs, nOCN-200/CNTs-12h and nOCN-200/CNTs-18h were 2.74, 2.57 and 2.45, respectively, at the reaction potential
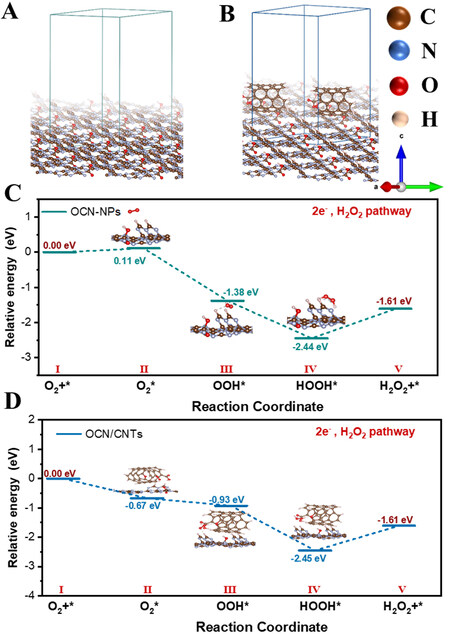
DFT calculations were carried out to gain insights into the mechanism. The optimized structural models of the OCN-NPs and OCN-200/CNTs-18h are illustrated in Figure 9A and B, respectively. The CNTs interacted with the OCN-NPs via the π-π conjugation. The relative Gibbs free energies of the reaction intermediates of the ORR to H2O2 are shown in Figure 9C and D. O2 was adsorbed as an adsorption state (O2*) on the catalyst surface, which was then converted to an intermediate state (OOH*) with free hydrogen in the aqueous solution. Subsequently, the intermediate state (OOH*) could undergo a proton coupling process with water molecules to form an adsorbed state HOOH*, finally desorbing into H2O2[48,50]. In particular, the formation of the adsorption state (O2*) on OCN-200/CNTs-18h was a spontaneous reaction corresponding to the ΔG ~ -0.67 eV. However, this process of OCN-NPs was a non-spontaneous process due to the endothermic ΔG ~0.11 eV and required a large activation energy barrier. This result indicated that the OCN-200/CNTs-18h electrode could thermodynamically preferentially facilitate the oxygen reduction to H2O2 with a lower Gibbs free energy of adsorbed oxygen in comparison with OCN-NPs. The TPD confirmed the DFT results, as shown in Figure 8F, with OCN-200/CNTs-18h exhibiting excellent chemical adsorption properties of O2. Based on the above experimental results, we believe that OCN-200/CNTs-18h is conducive to the two-electron ORR.
CONCLUSIONS
In summary, we developed a novel nanocomposite by threading OCN-NPs with CNTs through a controlled assembly. As an electrode material, it exhibited high efficiency in photo-coupled electrocatalytic H2O2 production through the 2e-ORR with the optimum H2O2 yield and faradaic H2O2 efficiency reaching up to 30.7 mmol/gcat/h and 95%, respectively. Furthermore, it also displayed strong durability in a long reaction period. The high catalytic activity could be attributed to the unique structure of OCN/CNTs, which promoted the exposure of catalytic sites and facilitated the carrier separation and transfer. More importantly, the application of a light field significantly improved electron transport efficiency and enhanced the selectivity toward H2O2 production by the 2e-ORR pathway. This work provides an important platform for developing high-performance carbon-based nanocomposites as metal-free photo-coupled electrocatalysts for the 2e-ORR to produce H2O2, which may offer more opportunities for industrial applications.
DECLARATIONS
AcknowledgmentsThis paper would like to acknowledge the teachers from the Experimental Analysis Center of Shanghai Normal University, as well as the partners of our research group.
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Zhu Q, Fan J, Li G
Performed data acquisition, as well as provided administrative, technical, and material support: Tao Y, Shang H, Xu J, Zhang D, Li H
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China (Grant No. 21876113, 22176127, 22022608, and 21261140333) and the Singapore National Research Foundation under Grant No. NRF2017NRF-NSFC001-007. This work was also sponsored by Shanghai Rising-Star Program (No. 19QA1404100), Science and Technology Commission of Shanghai Municipality (No. 20ZR1421400) and Shanghai Engineering Research Center of Green Energy Chemical Engineering.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Serra-maia R, Michel FM, Kang Y, Stach EA. Decomposition of hydrogen peroxide catalyzed by AuPd nanocatalysts during methane oxidation to methanol. ACS Catal 2020;10:5115-23.
2. Chen H, Huang M, Yan W, Bai W, Wang X. Enzymatic regio- and enantioselective C-H oxyfunctionalization of fatty acids. ACS Catal 2021;11:10625-30.
3. Teng Z, Yang N, Lv H, et al. Edge-functionalized g-C3N4 nanosheets as a highly efficient metal-free photocatalyst for safe drinking water. Chem 2019;5:664-80.
4. Hwang GB, Huang H, Wu G, et al. Photobactericidal activity activated by thiolated gold nanoclusters at low flux levels of white light. Nat Commun 2020;11:1207.
5. Cai J, Huang J, Wang S, et al. Crafting mussel-inspired metal nanoparticle-decorated ultrathin graphitic carbon nitride for the degradation of chemical pollutants and production of chemical resources. Adv Mater 2019;31:e1806314.
6. Zhao H, Chen Y, Peng Q, Wang Q, Zhao G. Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and OH generation in solar photo-electro-Fenton process. Appl Catal B 2017;203:127-37.
7. Zong X, Chen H, Seger B, et al. Selective production of hydrogen peroxide and oxidation of hydrogen sulfide in an unbiased solar photoelectrochemical cell. Energy Environ Sci 2014;7:3347-51.
8. Shi X, Zhang Y, Siahrostami S, Zheng X. Light-driven BiVO4 -C fuel cell with simultaneous production of H2O2. Adv Energy Mater 2018;8:1801158.
9. Mase K, Yoneda M, Yamada Y, Fukuzumi S. Seawater usable for production and consumption of hydrogen peroxide as a solar fuel. Nat Commun 2016;7:11470.
10. Liu N, Han M, Sun Y, et al. Ag-C3N4 based photoelectrochemical cell using O2/H2O redox couples. Energy Environ Sci 2018;11:1841-7.
11. Liu J, Zou Y, Jin B, Zhang K, Park JH. Hydrogen peroxide production from solar water oxidation. ACS Energy Lett 2019;4:3018-27.
12. Campos-Martin JM, Blanco-Brieva G, Fierro JL. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew Chem Int Ed Engl 2006;45:6962-84.
13. Kim HW, Bukas VJ, Park H, et al. Mechanisms of two-electron and four-electron electrochemical oxygen reduction reactions at nitrogen-doped reduced graphene oxide. ACS Catal 2020;10:852-63.
14. Zhang T, Sun L, Sun X, Dong H, Yu H, Yu H. Radical and non-radical cooperative degradation in metal-free electro-Fenton based on nitrogen self-doped biochar. J Hazard Mater 2022;435:129063.
15. He C, Sankarasubramanian S, Ells A, et al. Self-anchored platinum-decorated antimony-doped-tin oxide as a durable oxygen reduction electrocatalyst. ACS Catal 2021;11:7006-17.
16. Siahrostami S, Verdaguer-casadevall A, Karamad M, et al. Erratum: enabling direct H2O2 production through rational electrocatalyst design. Nature Mater 2014;13:213-213.
17. Flaherty DW. Direct synthesis of H2O2 from H2 and O2 on Pd catalysts: current understanding, outstanding questions, and research needs. ACS Catal 2018;8:1520-7.
18. Chen Y, Gao R, Ji S, et al. Atomic-level modulation of electronic density at cobalt single-atom sites derived from metal-organic frameworks: enhanced oxygen reduction performance. Angew Chem Int Ed Engl 2021;60:3212-21.
19. Melchionna M, Fornasiero P, Prato M. The rise of hydrogen peroxide as the main product by metal-free catalysis in oxygen reductions. Adv Mater 2019;31:e1802920.
20. Li BQ, Zhao CX, Liu JN, Zhang Q. Electrosynthesis of hydrogen peroxide synergistically catalyzed by atomic Co-Nx-C sites and oxygen functional groups in noble-metal-free electrocatalysts. Adv Mater 2019;31:e1808173.
21. Jiang K, Back S, Akey AJ, et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat Commun 2019;10:3997.
22. Lu Z, Chen G, Siahrostami S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat Catal 2018;1:156-62.
23. Lim JS, Kim JH, Woo J, et al. Designing highly active nanoporous carbon H2O2 production electrocatalysts through active site identification. Chem 2021;7:3114-30.
24. Wang W, Tao Y, Fan J, et al. Fullerene-graphene acceptor drives ultrafast carrier dynamics for sustainable CdS photocatalytic hydrogen evolution. Adv Funct Materials 2022;32:2201357.
25. Dong K, Liang J, Wang Y, et al. Honeycomb carbon nanofibers: a superhydrophilic O2 -entrapping electrocatalyst enables ultrahigh mass activity for the two-electron oxygen reduction reaction. Angew Chem Int Ed Engl 2021;60:10583-7.
26. San Roman D, Krishnamurthy D, Garg R, et al. Engineering three-dimensional (3D) out-of-plane graphene edge sites for highly selective two-electron oxygen reduction electrocatalysis. ACS Catal 2020;10:1993-2008.
27. Sun Y, Sinev I, Ju W, et al. Efficient electrochemical hydrogen peroxide production from molecular oxygen on nitrogen-doped mesoporous carbon catalysts. ACS Catal 2018;8:2844-56.
28. Wu Y, Gao Z, Feng Y, et al. Harnessing selective and durable electrosynthesis of H2O2 over dual-defective yolk-shell carbon nanosphere toward on-site pollutant degradation. Appl Catal B 2021;298:120572.
29. Zhao X, Lei Y, Fang P, et al. Piezotronic effect of single/few-layers MoS2 nanosheets composite with TiO2 nanorod heterojunction. Nano Energy 2019;66:104168.
30. Zhang Y, Pan D, Tao Y, et al. Photoelectrocatalytic reduction of CO2 to syngas via SnOx -enhanced Cu2O nanowires photocathodes. Adv Funct Mater 2022;32:2109600.
31. Yan J, Wang Y, Zhang Y, Xia S, Yu J, Ding B. Direct magnetic reinforcement of electrocatalytic ORR/OER with electromagnetic induction of magnetic catalysts. Adv Mater 2021;33:e2007525.
32. Yuan S, Li Y, Peng J, et al. Conversion of methane into liquid fuels-bridging thermal catalysis with electrocatalysis. Adv Energy Mater 2020;10:2002154.
33. Shen Q, Chen Z, Huang X, Liu M, Zhao G. High-yield and selective photoelectrocatalytic reduction of CO2 to formate by metallic copper decorated Co3O4 nanotube arrays. Environ Sci Technol 2015;49:5828-35.
34. Yang D, Yu H, He T, et al. Visible-light-switched electron transfer over single porphyrin-metal atom center for highly selective electroreduction of carbon dioxide. Nat Commun 2019;10:3844.
35. Lu M, Zhang M, Liu CG, et al. Stable dioxin-linked metallophthalocyanine covalent organic frameworks (COFs) as photo-coupled electrocatalysts for CO2 reduction. Angew Chem Int Ed Engl 2021;60:4864-71.
36. Yang H, Yang D, Zhou Y, Wang X. Polyoxometalate interlayered zinc-metallophthalocyanine molecular layer sandwich as photocoupled electrocatalytic CO2 reduction catalyst. J Am Chem Soc 2021;143:13721-30.
37. Zhong H, Zhang Q, Wang J, et al. Engineering ultrathin C3N4 quantum dots on graphene as a metal-free water reduction electrocatalyst. ACS Catal 2018;8:3965-70.
38. Chen Z, Zhao J, Cabrera CR, Chen Z. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction. Small Methods 2019;3:1800368.
39. Pei Z, Zhao J, Huang Y, et al. Toward enhanced activity of a graphitic carbon nitride-based electrocatalyst in oxygen reduction and hydrogen evolution reactions via atomic sulfur doping. J Mater Chem A 2016;4:12205-11.
40. Zhao X, Pan D, Chen X, et al. g-C3N4 photoanode for photoelectrocatalytic synergistic pollutant degradation and hydrogen evolution. Appl Surf Sci 2019;467-468:658-65.
41. Kofuji Y, Isobe Y, Shiraishi Y, et al. Carbon nitride-aromatic diimide-graphene nanohybrids: metal-free photocatalysts for solar-to-hydrogen peroxide energy conversion with 0.2% efficiency. J Am Chem Soc 2016;138:10019-25.
42. Shiraishi Y, Kofuji Y, Sakamoto H, Tanaka S, Ichikawa S, Hirai T. Effects of surface defects on photocatalytic H2O2 production by mesoporous graphitic carbon nitride under visible light irradiation. ACS Catal 2015;5:3058-66.
43. Chu C, Zhu Q, Pan Z, et al. Spatially separating redox centers on 2D carbon nitride with cobalt single atom for photocatalytic H2O2 production. Proc Natl Acad Sci USA 2020;117:6376-82.
44. Liu Y, Li Q, Lian Z, et al. Polarization field promoted photoelectrocatalysis for synergistic environmental remediation and H2 production. Chem Eng J 2022;437:135132.
45. Zeng X, Liu Y, Kang Y, et al. Simultaneously tuning charge separation and oxygen reduction pathway on graphitic carbon nitride by polyethylenimine for boosted photocatalytic hydrogen peroxide production. ACS Catal 2020;10:3697-706.
46. Hou H, Zeng X, Zhang X. Production of hydrogen peroxide by photocatalytic processes. Angew Chem Int Ed Engl 2020;59:17356-76.
47. Desalegn BZ, Jadhav HS, Seo JG. Highly efficient g-C3N4 nanorods with dual active sites as an electrocatalyst for the oxygen evolution reaction. ChemCatChem 2019;11:2870-8.
48. Zhang P, Tong Y, Liu Y, et al. Heteroatom dopants promote two-electron O2 reduction for photocatalytic production of H2O2 on polymeric carbon nitride. Angew Chem Int Ed Engl 2020;59:16209-17.
49. Wang H, Jia J, Song P, et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: a step towards the electrochemical CO2 refinery. Angew Chem Int Ed Engl 2017;56:7847-52.
50. Wei Z, Liu M, Zhang Z, Yao W, Tan H, Zhu Y. Efficient visible-light-driven selective oxygen reduction to hydrogen peroxide by oxygen-enriched graphitic carbon nitride polymers. Energy Environ Sci 2018;11:2581-9.
51. Dan R, Chen W, Xiao Z, et al. N-doped biomass carbon/reduced graphene oxide as a high-performance anode for sodium-ion batteries. Energy Fuels 2020;34:3923-30.
52. Shen X, Xiao F, Zhao H, et al. In situ-formed PdFe nanoalloy and carbon defects in cathode for synergic reduction-oxidation of chlorinated pollutants in electro-fenton process. Environ Sci Technol 2020;54:4564-72.
53. Zhang D, Liu T, Yin K, Liu C, Wei Y. Selective H2O2 production on N-doped porous carbon from direct carbonization of metal organic frameworks for electro-Fenton mineralization of antibiotics. Chem Eng J 2020;383:123184.
54. Watzele S, Hauenstein P, Liang Y, et al. Determination of electroactive surface area of Ni-, Co-, Fe-, and Ir-based oxide electrocatalysts. ACS Catal 2019;9:9222-30.
55. Jakešová M, Apaydin DH, Sytnyk M, et al. Hydrogen-Bonded organic semiconductors as stable photoelectrocatalysts for efficient hydrogen peroxide photosynthesis. Adv Funct Mater 2016;26:5248-54.
56. Wang M, Dong X, Meng Z, et al. An efficient interfacial synthesis of two-dimensional metal-organic framework nanosheets for electrochemical hydrogen peroxide production. Angew Chem Int Ed Engl 2021;60:11190-5.
57. Hu J, Wang S, Yu J, Nie W, Sun J, Wang S. Duet Fe3C and FeNx sites for H2O2 generation and activation toward enhanced electro-fenton performance in wastewater treatment. Environ Sci Technol 2021;55:1260-9.
58. Wang W, Tao Y, Du L, et al. Femtosecond time-resolved spectroscopic observation of long-lived charge separation in bimetallic sulfide/g-C3N4 for boosting photocatalytic H2 evolution. Appl Catal B 2021;282:119568.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhu Q, Fan J, Tao Y, Shang H, Xu J, Zhang D, Li G, Li H. Photo-coupled electrocatalytic oxygen reduction to hydrogen peroxide using metal-free CNT-threaded oxidized g-C3N4. Energy Mater 2022;2:200029. http://dx.doi.org/10.20517/energymater.2022.33
AMA Style
Zhu Q, Fan J, Tao Y, Shang H, Xu J, Zhang D, Li G, Li H. Photo-coupled electrocatalytic oxygen reduction to hydrogen peroxide using metal-free CNT-threaded oxidized g-C3N4. Energy Materials. 2022; 2(4): 200029. http://dx.doi.org/10.20517/energymater.2022.33
Chicago/Turabian Style
Zhu, Qiong, Jinchen Fan, Ying Tao, Huan Shang, Jingcheng Xu, Dieqing Zhang, Guisheng Li, Hexing Li. 2022. "Photo-coupled electrocatalytic oxygen reduction to hydrogen peroxide using metal-free CNT-threaded oxidized g-C3N4" Energy Materials. 2, no.4: 200029. http://dx.doi.org/10.20517/energymater.2022.33
ACS Style
Zhu, Q.; Fan J.; Tao Y.; Shang H.; Xu J.; Zhang D.; Li G.; Li H. Photo-coupled electrocatalytic oxygen reduction to hydrogen peroxide using metal-free CNT-threaded oxidized g-C3N4. Energy Mater. 2022, 2, 200029. http://dx.doi.org/10.20517/energymater.2022.33
About This Article
Copyright
Data & Comments
Data
 Cite This Article 20 clicks
Cite This Article 20 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.