Multi-functional engineering of rare earth-based catalysts for high-efficiency water splitting
Abstract
Against the backdrop of global carbon neutrality goals, hydrogen production via electrochemical water splitting has emerged as a promising pathway for sustainable energy conversion. However, the sluggish kinetics of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) necessitate highly efficient electrocatalysts. While noble metal-based catalysts exhibit superior performance, their high cost and scarcity impede large-scale application. Rare earth elements (REEs), with their unique 4f electronic configurations, variable oxidation states, and strong oxygen affinity, offer exceptional potential for modulating the electronic and geometric structures of electrocatalysts. This review comprehensively summarizes recent advances in the application of REEs in electrocatalytic water splitting, with a focus on HER and OER. Key strategies such as doping, defect engineering, alloying, heterostructure construction and other strategies are discussed, highlighting how REEs enhance catalytic activity, stability, and bifunctional performance across acidic, alkaline, and neutral media. Specific attention is given to La-, Ce-, and Er-based catalysts, which demonstrate performance comparable to or surpassing that of noble metal benchmarks. This review summarizes recent progress in rare earth-based electrocatalysis and highlights key challenges such as sustainability, resource utilization, and mechanistic understanding. It emphasizes the role of strategies such as doping, defect, alloy, and heterostructure engineering in optimizing activity and stability. By integrating in situ characterization with data-driven design, it provides guidance for developing next-generation, eco-friendly REE-based catalysts for green hydrogen production.
Keywords
INTRODUCTION
In light of global carbon peaking and carbon neutrality goals, the transition from fossil fuels to clean and renewable energy sources has become both inevitable and strategically essential. Among emerging energy carriers, hydrogen stands out for its high energy density and zero-carbon utilization, positioning it as a key vector for green energy applications[1,2]. Electrochemical water splitting, which directly produces high-purity hydrogen from water, is widely regarded as a scalable and sustainable pathway to meet future hydrogen demands[3,4]. However, its efficiency is constrained by the sluggish kinetics of the hydrogen (HER) and oxygen evolution reactions (OER), both involving complex multi-electron transfer processes and high overpotentials[5,6]. While noble metal-based catalysts [Pt/C for HER and IrO2 or ruthenium oxide (RuO2) for OER] demonstrate excellent activity and stability, their scarcity and high cost severely limit large-scale implementation. As a result, the development of low-cost, efficient, and durable non-precious metal electrocatalysts has become a critical research focus in advancing water electrolysis technologies toward practical application[7-9].
In recent years, transition metal (TM)-based materials including Fe, Co, Ni and their oxides, carbides, sulfides, and phosphides have garnered significant interest owing to their earth abundance, tunable structures, and potential catalytic activity[10,11]. However, these materials still suffer from several inherent limitations, including unsatisfactory electrical conductivity, limited exposure of active sites, and insufficient long-term stability under harsh electrochemical conditions. To overcome these challenges, researchers have increasingly turned to novel strategies that can complement or surpass the performance of TMs.
In this context, rare earth elements (REEs) have attracted considerable attention as a promising class of modifiers and active components in electrocatalysis. Compared with noble metals such as platinum and iridium oxide, which as of 2025 are priced at approximately $30-35 g-1 and over $500 g-1, respectively, common rare-earth oxides such as lanthanum oxide (La2O3), cerium dioxide (CeO2), and erbium oxide (Er2O3) are markedly less expensive, at around $557, $1,374, and $41,403 t-1, respectively. This vast price disparity arises from the higher abundance and lower extraction cost of REEs, though their markets remain sensitive to supply chain and geopolitical fluctuations[12]. Economically and structurally, REEs possess partially filled 4f orbitals, variable oxidation states, and strong oxygen affinity[13]. These distinctive electronic and structural characteristics provide unique opportunities for catalyst design, enabling precise tuning of electronic structures, modulation of intermediate adsorption behavior, introduction of beneficial surface defects, and improvement of structural stability[14-16]. Consequently, incorporating REEs into conventional catalytic systems, or directly employing REE-based catalysts, has proven effective in overcoming the intrinsic limitations of traditional TM-based materials, leading to remarkable improvements in both HER and OER activity and durability[17,18]. Building upon these intrinsic merits, researchers have proposed a series of strategies to further optimize the utilization of REEs and maximize catalytic performance[19]. Among them, element doping represents one of the earliest and most extensively studied approaches. Incorporating REEs into host lattices can effectively tailor the electronic structure and induce lattice strain, thereby altering the adsorption behavior of key intermediates. On the one hand, the relatively high electronegativity of REE ions can modulate the electron density around adjacent metal centers, optimizing the adsorption free energy of catalytic intermediates. On the other hand, the mismatch in ionic radii between REEs and host atoms introduces lattice distortions, which increase the exposure of active sites while improving structural robustness. For instance, doping with La or Nd has been shown to strongly interact with TMs, inducing a shift in the d-band center and consequently weakening the binding strength of hydrogen and oxygen intermediates, thus accelerating both HER and OER kinetics[20]. Beyond doping, constructing heterostructures that incorporate REEs has emerged as another powerful strategy to overcome the kinetic limitations of conventional water-splitting catalysts. Through interfacial electronic coupling and band alignment, REE-based heterostructures can promote charge redistribution across interfaces, stabilize high-valence active species, and facilitate key reaction steps that are otherwise sluggish in single-phase systems[21-23]. In parallel, the application of REEs in single-atom catalysts (SACs) has opened exciting opportunities for atomic-level catalyst design. Isolated rare earth atoms can precisely modulate the electronic environment of adjacent metal centers, either through charge redistribution or by lowering the local electron density, thereby tailoring the configuration of active sites and significantly boosting catalytic activity. Finally, defect engineering of REE-based oxides has emerged as a versatile and effective strategy to enhance water-splitting performance. Rare-earth oxides (e.g., CeO2) exhibit relatively low formation energies for oxygen vacancies, enabling the introduction of abundant lattice defects through approaches such as thermal treatment, atmosphere control, plasma induction, or chemical etching. These structural defects not only enhance the electrical conductivity of the materials but also modulate the adsorption and activation of key reaction intermediates, thereby facilitating rate-determining steps and markedly improving overall catalytic performance[24,25].
Owing to their unique electronic tunability and multi-functional catalytic mechanisms, REEs confer unparalleled potential to overcome the intrinsic performance limitations of conventional electrocatalytic materials. Rare earth-based electrocatalysts are thus highly promising both as catalytic promoters in electrochemical reactions and as standalone active materials[26,27]. Motivated by this potential, the present review systematically highlights recent advances in the application of REEs in electrocatalysis, with a particular emphasis on their roles in the HER and OER, as well as strategies for material design and performance optimization. By providing a comprehensive overview of fundamental principles, design strategies, and practical considerations, this review aims to offer theoretical guidance and technical insights for the rational development of efficient rare earth-based catalysts, thereby facilitating the advancement of clean energy technologies toward higher efficiency, lower cost, and enhanced sustainability.
REACTION MECHANISM OF WATER ELECTROLYSIS
HER
HER is a half-reaction that occurs at the cathode during electrochemical water splitting. Depending on the electrolyte conditions, the HER proceeds via two possible pathways: Volmer-Tafel and Volmer-Heyrovsky mechanisms [Equations (1)-(6)]:
In acidic media, the reaction pathway is given by[28]:
Volmer step:
Heyrovsky step:
Tafel step:
In alkaline and neutral media, the reaction pathway follows[29]:
Volmer step:
Heyrovsky step:
Tafel step:
OER
OER is the half-reaction that occurs at the anode during electrochemical water splitting. Depending on the electrolyte conditions, from a theoretical perspective, Nørskov proposed the following four-step reaction pathway under acidic conditions, as given in[30]:
In alkaline media, the reaction pathway is expressed by[31]:
PROPERTIES OF RARE RARTH ELEMENTS
The discovery of REEs dates back to the late 18th century. The first REE was identified by Finnish chemist Johan Gadolin in 1794, when he isolated yttria (Y2O3) from a dark mineral sample. This mineral had been discovered earlier, in 1787, by amateur mineralogist Carl Arrhenius in the village of Ytterby near Stockholm, Sweden[27, 32, 33]. As shown in Figure 1A, REEs are typically categorized into two groups based on their atomic numbers and physicochemical properties: light REEs [LREEs, lanthanum (La)-europium (Eu)] and heavy REEs [HREEs, gadolinium (Gd)-lutetium (Lu), including Y][34].
Figure 1. (A) Rare earth elements (REEs) in the periodic table[45]; (B) World reserves of REEs by principal countries.
The term “rare earth” originates not from their actual scarcity, but from the historical difficulty in separating and purifying these elements[35]. According to the 2025 report by the U.S. Geological Survey (USGS), global REE reserves are estimated at 90 million metric tons. China leads the world in REE reserves with 44 million tons, accounting for 48.9% of the global total, followed by Brazil (21 million tons, 23.3%) and India (6.9 million tons, 7.6%). Australia, Russia, and Vietnam hold approximately 6.3%, 4.2%, and 3.8% of the total reserves, respectively [Figure 1B]. Chemically, REEs are characterized by a stable +3 oxidation state, high reactivity, small variations in electronegativity, and weak ligand field effects. Due to the lanthanide contraction, REEs possess similar ionic radii, and their 4f electrons are generally shielded and do not participate in bonding. These features allow REEs to form a wide range of compounds and enable their versatile applications in photo-, electronic-, and magneto-functional systems[36].
From a catalytic perspective, REEs play a dual role as both structural modifiers and electronic regulators. Their low Pauling electronegativity allows them to act as electron donors within host lattices, promoting directional charge transfer from REE centers to TM sites. This charge redistribution optimizes the d-band center of TM active sites, thereby modulating the adsorption strength of key reaction intermediates and accelerating catalytic kinetics[37]. Meanwhile, the mismatch in ionic radii between REEs and TMs introduces local lattice strain and defects, altering TM-O bond lengths and facilitating the formation of oxygen vacancies. These vacancies not only serve as additional active sites but also act as electron reservoirs that promote the activation and dissociation of H2O or O2[38]. The unique 4f electronic configuration represents another defining feature of REEs. Shielded by outer-shell electrons, the 4f orbitals retain strong localization and weak interaction with surrounding ligands. When REEs couple with TM species, the localized 4f states induce electronic redistribution and interfacial polarization, effectively tuning the metal’s electronic structure and enhancing catalytic performance[39]. These mechanisms collectively endow REEs with remarkable potential in electrocatalysis, particularly in HER/OER, as well as in CO2 reduction and other redox processes[40,41]. Beyond catalysis, the versatile physicochemical properties of REEs underpin their wide-ranging industrial applications. In metallurgy, they act as alloying and modifying agents to enhance mechanical and thermal properties; in medicine, they serve as contrast agents in bio-imaging and as carriers in anti-inflammatory drug delivery systems[42]; in technology, they are key components of data storage devices and hard disk drives[43]; in clean energy, they are indispensable in high-efficiency wind turbines and electric vehicle powertrains; and in defense, they are vital for precision-guided weapons and phased-array radar systems[44]. Collectively, these multifaceted roles highlight the scientific and strategic significance of REEs across modern industries and energy technologies.
STRATEGIES FOR ENHANCING THE ACTIVITY OF RARE EARTH ELEMENT-BASED WATER ELECTROLYSIS CATALYSTS
To systematically enhance the catalytic performance of rare earth-based electrocatalysts, a comprehensive understanding of their structural and electronic properties is essential. The intrinsic activity is governed not only by the electronic configuration of the active sites but also by the surface coordination environment, defect states, and interfacial interactions with surrounding components. Accordingly, various rational design strategies have been developed to modulate these factors at the atomic and nanoscale levels[46]. Among them, doping has been widely employed to tune the electronic structure and optimize the adsorption of reaction intermediates, while defect engineering focuses on creating active sites and adjusting electronic and interfacial energy landscapes. Alloying, on the other hand, leverages charge redistribution and lattice strain to fine-tune catalytic kinetics, and the construction of heterostructures promotes interfacial charge transfer, electronic reconstruction, and synergistic enhancement of reaction pathways[39,47]. In the following sections, we discuss these strategies in detail, highlighting their mechanisms, representative examples, and design principles that enable the development of high-performance rare earth-based electrocatalysts.
Doping
Doping is a widely adopted strategy to enhance the catalytic activity of electrocatalysts. The main objective is to regulate the electronic structure of the parent material by introducing heteroatoms. Such modifications can optimize the adsorption free energy of active sites and reaction intermediates, while significantly improving charge transport efficiency, thereby accelerating catalytic reaction kinetics[48,49]. In recent years, REE doping has emerged as a powerful method for tuning the crystal structure and electronic state at the atomic scale, offering new approaches to overcoming these limitations. For example, Fu et al. proposed a TM doping strategy by incorporating Fe into LaNiO3 nanocrystals[50]. The resulting Fe-doped LaNiO3 exhibited significantly enhanced OER activity in 1 M KOH. The introduction of Fe not only induced lattice distortion in LaNiO3 and increased oxygen vacancy concentration, but also elevated the charge density on Ni sites, thereby promoting *OOH intermediate formation and facilitating O-O bond cleavage [Figure 2A]. Further investigation into the structure-activity relationship and underlying mechanism revealed that Fe4+ doping in LaNiO3 enhances the rate-determining O → OH step in the OER process by facilitating deprotonation through its unique electronic properties [Figure 2B]. This study demonstrates that doping TMs into a rare earth-based host material can synergistically modulate its electronic structure and surface reactivity.
Figure 2. (A) Schematic model of epitaxial growth of Sr and Fe-doped LaNiO3 perovskite thin films on STO (001) substrate; (B) Schematic representation of the electronic coupling between Ni3+ and Fe4+ in SrLNFO-0.2. This figure is quoted with permission from Fu et al.[50]; (C) HAADF-STEM image and elemental mappings of La-RuO2. This figure is quoted with permission from Zhu et al.[54]; (D) HER polarization curves of CeNiFe-MOF‖CeNiFe-MOF and Pt/C‖IrO2 for water electrolysis; (E) OER polarization curves; (F) The free energy diagram for the HER process. This figure is quoted with permission from Yang et al.[55]. STO: SrTiO3; HAADF-STEM: high-angle annular dark-field scanning transmission electron microscopy; MOF: metal-organic framework; OER: oxygen evolution reaction; HER: hydrogen evolution reaction.
Beyond their role as host materials, REEs are also frequently employed as functional additives in other electrocatalysts. In these roles, they modulate the electronic structure, interfacial adsorption behavior, or reaction pathways of intermediates, further enhancing the overall synergistic effect in electrocatalytic processes[51]. Unlike bulk doping, these strategies emphasize the auxiliary role of rare earth ions at interfaces or in heterostructures, for example, by inducing surface reconstruction, adjusting band gaps, or providing electron buffering zones. Thus, rare earth ions not only participate in electron transfer processes but also contribute to interface engineering and reaction coordination[52,53]. For instance, La doping has been shown to introduce oxygen vacancies and modulate the surface electronic structure of RuO2, enhancing electron accumulation and accelerating charge transfer [Figure 2C], which in turn significantly improves its electrocatalytic activity for water splitting[54]. Similarly, Yang et al. doped cerium (Ce) into a NiFe-metal-organic framework (MOF) catalyst to construct a CeNiFe-MOF/nickel foam (NF) system with synergistic electronic effects[55]. Under alkaline conditions, this material demonstrated markedly improved performance for both HER and OER (HER: 113 mV; OER: 224 mV at 10 mA cm-2), and exhibited excellent stability in both freshwater and simulated seawater environments [Figure 2D and E]. In situ X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) calculations revealed that Ce doping induced redistribution of electron density in the Ni-Fe framework, facilitated dynamic adsorption-desorption of key intermediates (e.g., H*, OH*), and formed a multiphase interfacial synergy mechanism that significantly lowered reaction energy barriers [Figure 2F]. Compared to traditional bimetallic systems, the incorporation of rare earth ions endows the catalyst with superior charge transfer capability and faster intermediate response, highlighting their immense potential as cocatalysts.
Currently, the study of doping strategies in rare earth-based materials remains in a stage of rapid development. Key challenges include understanding the effects of doping type (n-type/p-type), dopant concentration, spatial site selection, and the cooperative mechanisms at heterointerfaces. Moving forward, the integration of multiscale computational modeling with in situ electrochemical and spectroscopic characterization is expected to unveil the microscopic roles of dopants in dynamic reaction environments, thereby advancing the application of rare earth-based catalysts in clean energy conversion systems.
Defect engineering
In addition to doping regulation, defect engineering has emerged as another crucial structural tuning strategy with great potential for enhancing the performance of rare earth-based catalysts. Due to their variable valence states and highly coordinated crystal structures, REEs are prone to the formation of oxygen vacancies, lattice distortions, and non-stoichiometric coordination environments under reaction
Huang et al. employed an atmosphere-controlled strategy by annealing Fe2O3-CeO2 in a reducing Ar atmosphere, successfully inducing the formation of abundant and stable oxygen vacancies on the surface [Figure 3A][60]. This treatment enhanced both electronic conductivity and intermediate adsorption capacity. The resulting Fe2O3-CeO2 nanoparticles exhibited significantly superior performance in alkaline OER compared to conventional CeO2. The performance enhancement was attributed to the preferential formation of oxygen vacancies on the CeO2 surface, which increased the Ce3+ ratio and optimized the electronic structure of Fe2O3 through charge transfer. This strengthened the interfacial interaction between Fe2O3 and CeO2, facilitating electron transfer from CeO2 to Fe2O3, thereby promoting O2 desorption and markedly boosting the intrinsic catalytic activity.
Figure 3. (A) Schematic illustration of the synthetic process for Fe2O3@CeO2-OV. This figure is quoted with permission from Huang et al.[60]; (B) Schematic diagram of the fabrication procedures; (C) LSV curves. This figure is quoted with permission from Wang et al.[61]; (D) The schematic synthesis process of BiLaO-3 catalysts. This figure is quoted with permission from Wang et al.[62]. LSV: Linear sweep voltammetry.
In addition to atmosphere control, plasma-induced defect engineering has emerged as a popular strategy in recent years. As shown in Figure 3B, Wang et al. employed a low-temperature NH3/Ar plasma treatment to modify the surface of CeO2 nanosheets, introducing abundant shallow defects without damaging the bulk lattice[61]. This significantly enhanced the HER activity, reducing the overpotential to 65 mV at 10 mA cm-2 [Figure 3C]. The plasma treatment effectively modulated the surface charge distribution and exposed more active sites, thereby promoting the adsorption/desorption of H* intermediates. Thermal treatment-induced structural reconstruction has also been widely applied in rare earth-based catalysts. As depicted in Figure 3D, Zou et al. synthesized oxygen-vacancy-rich La2O3/C composites through high-temperature pyrolysis of MOF precursors[62]. The resulting catalysts exhibited excellent bifunctional HER/OER performance in alkaline media. Thermal treatment not only enhanced the electronic coupling between the carbon layer and rare-earth oxides, but also induced the formation of defect-rich layers around rare-earth sites, improving interfacial conductivity and the density of active sites. The resulting catalyst exhibited a current density of more than 200 mA cm-2 at -1.06 V [vs. Reversible Hydrogen Electrode (RHE)].
Comprehensive studies have shown that defect engineering enhances the catalytic performance of rare earth-based catalysts through a dual mechanism: on the one hand, it introduces abundant active centers, and on the other, it modulates the electronic structure and interfacial energy states to optimize the reaction process both thermodynamically and kinetically. Specifically, various defect-engineering strategies not only create additional catalytic sites but also reconstruct the electronic configuration, enhance charge carrier mobility, and regulate the binding energies of reaction intermediates. These synergistic effects underscore the critical role of defect engineering in the development of high-performance electrocatalytic systems.
Alloy
Alloys are materials composed of two or more chemical elements, with at least one being a metal. In rare-earth alloys, charge transfer induced by metallic bonding not only significantly tunes the electronic band structure and catalytic activity of the catalyst, but also reshapes the spatial distribution of active components through geometric configuration changes such as lattice strain modulation and lattice constant alteration[63,64]. Alloying REEs further allows for precise regulation of the microstructure evolution and phase distribution of the metallic matrix, making it an effective strategy for optimizing the performance of rare earth-based catalysts[65]. Compared with late TM systems, rare-earth intermetallic compounds exhibit strong d-f orbital coupling, resulting in a negative enthalpy of alloy formation. This provides an intrinsic driving force for reducing lattice reconstruction energy barriers and stabilizing metastable phases thermodynamically[66]. As shown in Figure 4A, Zhang et al. synthesized Rh3Sc/Y nanostructured alloys via a sodium vapor reduction method[67]. Compared to Rh/C, all X-ray diffraction (XRD) peaks of Rh3Sc/C and Rh3Y/C shift to higher 2θ angles, indicating an increase in alloy lattice spacing and the formation of Rh-Sc and Rh-Y alloys. Energy-dispersive X-ray spectroscopy (EDS) results show a uniform distribution of Rh and Sc/Y elements in the Rh3Sc/C and Rh3Y/C samples, confirming the formation of Rh-RE nanoalloys. Alloying active metal rhodium (Rh) with REEs scandium (Sc) and yttrium (Y) enabled directional charge transfer through metallic bonding, significantly regulating both the electronic structure and spatial configuration of the catalyst, thus synergistically enhancing the HER performance. The low electronegativity of REEs leads to electron enrichment at Rh active sites via a ligand effect, reducing the electron binding energy and increasing surface electron density. This weakens the adsorption of hydrogen intermediates (H*) and accelerates desorption kinetics. Meanwhile, the relatively large atomic radii of Sc and Y introduce considerable lattice strain, causing lattice expansion and shifting the d-band center of Rh downward, which further reduces the reaction energy barrier. Zhang et al. employed a solid-state synthesis strategy to fabricate ternary rare-earth Pt3-xIrxSc alloy nanoparticles [Figure 4B][68]. XRD characterization typically confirms the formation of well-defined cubic Pt-Sc alloy phases such as Pt3Sc, indicating effective alloying between Pt and Sc. Corresponding scanning electron microscopy (SEM) analyses often reveal surface structures dominated by the (100) crystal plane, where ordered Pt/Ir and Pt/Ir-Sc atomic arrangements are observed. Upon acid treatment, partial leaching of Sc can occur, resulting in a Pt/Ir-enriched noble metal surface. Such surface reconstruction has been reported to enhance electrochemical stability and corrosion resistance under acidic operating environments, thereby improving the long-term durability of Pt-based catalysts. The low electronegativity of Sc facilitates electron transfer from Sc to Pt and Ir, resulting in a negatively charged Pt surface. This charge redistribution downshifts the d-band center of Pt, weakens the adsorption energy of H* intermediates, and optimizes HER kinetics. Additionally, the strong orbital overlap between Sc and Pt/Ir reduces the alloy formation enthalpy and enhances structural stability, enabling excellent durability under acidic, neutral, and alkaline conditions. The optimized Pt2IrSc showed enhanced HER activity and stability across all pH values. To achieve a current density of 10 mA cm-2, it only required low overpotentials of 13 mV, 18 mV, and 25 mV in 0.5 M H2SO4, 1 M phosphate buffered saline (PBS), and 1 M KOH, respectively.
Figure 4. (A) Schematic illustration of the synthesis of Rh3RE alloys on Ketjen black. This figure is quoted with permission from Zhang et al.[67]; (B) Schematic illustration of the synthesis of Pt3-xIrxSc alloys on graphene. This figure is quoted with permission from Zhang et al.[68]; (C) Schematic diagram for the synthesis of the PdCeMoCuRu HEA. This figure is quoted with permission from Zhang et al.[64]. OPDA: o-Phenylenediamine; HEA: high-entropy alloy; NC: nanocrystal; RE: rare earth.
High-entropy alloys (HEAs), a recently emerging multi-metal construction strategy, have also been adopted to build rare-earth-involved synergistic electrocatalytic platforms. As illustrated in Figure 4C, Zhang et al. synthesized HEA nanoparticles comprising Ce, Ni, Fe, Co, and Cu, and used rapid annealing to induce microphase separation and lattice stress field optimization. In comparison with the reference XRD pattern, the PdCeMoCuRu HEA exhibits a slight shift of diffraction peaks toward higher angles, suggesting that Ce incorporation compresses the Pd-Pd interatomic spacing, resulting in lattice contraction and compressive strain, which may further trigger dynamic structural reconstruction under electrocatalytic conditions. The resulting catalyst exhibited an HER overpotential of 89 mV and an OER overpotential of 274 mV in 1 M KOH, with long-term stability at 500 mA cm-2 for over 100 h. The study revealed that Ce incorporation facilitates d-f orbital coupling, enhances electron transport efficiency, and generates internal electric fields via lattice distortion at the interface, thereby accelerating the reaction kinetics of H*/OH* intermediates[64].
Heterostructures
The construction of heterostructures has emerged as another vital approach to enhance the performance of rare earth-based electrocatalysts. By integrating rare-earth materials with highly active components such as TMs, metal oxides, or sulfides, heterojunction interfaces can be formed that effectively overcome inherent limitations in catalytic structure and activity[69]. These heterostructures often exhibit unique band bending effects, interfacial charge transfer pathways, and localized electric field modulation, which enable surface electronic reconstruction, increased active site density, and accelerated intermediate reaction kinetics, ultimately leading to synergistic enhancement in key reactions such as the HER and OER[70,71].
Song et al. designed a nitrogen-doped carbon encapsulated Co/CeO2/Co2P/CoP heterostructure (Co/CeO2/Co2P/CoP@NC) by embedding CeO2 nanowires into a zeolitic imidazolate framework-67 (ZIF-67) precursor followed by carbonization and phosphidation[72]. The strong interfacial coupling between CeO2 and Co2P/CoP facilitated interfacial electron transfer, which optimized the adsorption strength of key intermediates and reduced the kinetic barriers for both OER and HER [Figure 5A-C]. Similarly, Chen et al. synthesized a bifunctional electrocatalyst composed of a MoO2-CeOx heterostructure using interfacial engineering[73]. The heterointerface enabled efficient electron transfer and electronic structure modulation, accelerating overall charge transport. Additionally, the valence flexibility of Ce4+/Ce3+ enhanced electron mobility, while unoccupied orbital centers in MoO2 promoted favorable H* adsorption and desorption. As a result, the MoO2-CeOx/NF catalyst demonstrated outstanding HER activity [Figure 5D and F].
Figure 5. (A) TEM images of Co/CeO2/Co2P/CoP@NC heterostructures; (B) HER performance of LSV polarization curves of Co/CeO2/Co2P/CoP@NC heterostructures; (C) OER performance of LSV polarization curves. This figure is quoted with permission from Song et al.[72]; (D) TEM image of MoO2-CeOx/NF; (E) LSV curves of MoO2-CeOx/NF and Pt/C at 10.0 mA cm-2 in 0.5 M H2SO4; (F) PDOS programs of MoO2, Vo-MoO2, CeOx and Vo-MoO2-CeOx. This figure is quoted with permission from Chen et al.[73]; (G) TEM image of the CeO2@C60 core-shell hybrid at 200 nm; (H) LSV polarization curves of pure CeO2/SS, C60/SS and CeO2@C60/SS core-shell hybrid in 1.0 M KOH; (I) chronoamperometric curves. This figure is quoted with permission from Munawar et al.[74]. TEM: Transmission electron microscopy HER: hydrogen evolution reaction; LSV: linear sweep voltammetry; OER: oxygen evolution reaction; NF: nickel foam; PDOS: projected density of states.
Core-shell heterostructures further improve interface stability, corrosion resistance, and charge transport by coating rare-earth materials with active phases or protective outer layers. For instance, Munawar et al. employed ultrasonic assembly to fabricate a CeO2@C60 core-shell structure [Figure 5G][74]. The C60 shell formed a three-dimensional (3D) conductive network that significantly accelerated charge migration. Interfacial electronic coupling induced a Ce3+/Ce4+ mixed valence state and abundant oxygen vacancy defects, which together reduced the *OOH intermediate adsorption/desorption barrier. Consequently, the OER overpotential was lowered to 312 mV at 10 mA cm-2, and the catalyst exhibited excellent long-term stability, maintaining constant potential operation in 1 M KOH for over 45 h [Figure 5H and I].
Through rational design of heterostructured interfaces, it is possible to simultaneously improve active site utilization, enhance interfacial synergy, and optimize catalytic kinetics. This provides a multidimensional, tunable material platform for advancing HER, OER, and other critical electrocatalytic reactions.
Other strategies
Surface functionalization
Surface functionalization involves the selective modification of catalyst surfaces with REEs to enhance active site exposure, tune adsorption behavior, or improve stability, typically via techniques such as chemical grafting, atomic layer deposition (ALD), or surface coating, distinct from bulk doping, which alters the entire lattice structure. For instance, Flores-Melo et al. employed an electrochemical deposition strategy to anchor CeO2 nanoparticles onto NF, forming a CeO2-functionalized Ni surface that exhibited enhanced OER performance in alkaline media with an onset potential of 1.48 V, a potential of 1.56 V at 5 mA cm-2, and a Tafel slope of 75.71 mV dec-1[75]. The CeO2 layer promotes oxygen vacancy formation and facilitates *OOH intermediate adsorption without modifying the Ni bulk. However, long-term stability tests in an electrolyzer revealed instability and high overpotentials after 500 h at 0.5 A cm-2 in 15 wt.% NaOH, attributed to the low conductivity of CeO2 and retention of passive NiO/Ni(OH)2 layers. These examples highlight how surface functionalization leverages the 4f electronic properties of REEs to optimize interfacial kinetics while preserving substrate integrity. However, challenges remain in ensuring uniform coating and preventing delamination under prolonged operation.
MOF/COF Integration
MOF/covalent organic framework (COF) integration leverages the high porosity and tunable coordination environments of frameworks to incorporate REEs as nodes or dopants, thereby enhancing multiphase synergy and stability. MOFs are widely used in REE applications, whereas COFs, characterized by their rigid covalent bonds, are well-suited for harsh environments but encounter challenges in REE incorporation due to low solubility and framework compatibility[46]. For example, Li et al. developed an yttrium and cerium co-doped ultrathin Ni-MOF nanosheet array (NiYCe-MOF/NF) through a simple hydrothermal method on NF, which demonstrated exceptional alkaline water splitting performance with a HER overpotential of 136 mV and an OER overpotential of 245 mV at 10 mA cm-2, alongside a cell voltage of 1.54 V at 100 mA cm-2 in 1.0 M KOH[76]. The codoping of Y and Ce optimized electron transfer, active site exposure, and bimetallic synergistic effects. Another instance involves[77] Dy2O3/graphene/Gd-MOF ternary composites developed by Ma et al., where REE synergy effectively reduces the OER overpotential and enhances charge transfer. Within MOF/COF systems, the variable valence states of REEs facilitate oxygen vacancy formation, though framework collapse during synthesis remains a critical issue to address. Future exploration of REE-COFs holds promise for developing highly ordered structures that could improve selectivity in water splitting.
APPLICATION OF RARE EARTH-BASED CATALYSTS IN HER
REEs possess distinctive 4f electronic configurations, variable valence states, and flexible coordination environments, which can influence the electronic structure, surface properties, and interfacial interactions of electrocatalysts. These characteristics make REEs versatile components for tuning host materials, potentially affecting lattice structure, defect formation, and adsorption/desorption behavior of hydrogen intermediates. Given the diversity of REEs, their incorporation can lead to different structural features and catalytic behaviors depending on the specific element and host system. Accordingly, the following sections (Lanthanum-based - Other rare earth-based catalysts) discuss the application of rare earth-based catalysts in HER by focusing on individual elements (lanthanum (La), cerium (Ce), erbium (Er), and other emerging rare-earth metals) to highlight representative examples, structural characteristics, and mechanistic insights to clarify how each element contributes to HER performance.
Lanthanum-based
The 4f orbitals of Lanthanum can dynamically modulate the electron occupancy of the 5d orbitals and the charge distribution of neighboring atoms, thereby creating effective electron-accepting sites. This property significantly enhances the surface charge transfer capability of the material, leading to improved HER catalytic activity. Wei et al. synthesized a novel La-doped molybdenum phosphide nanoparticle (La-MoP@NC) encapsulated in a nitrogen-doped carbon matrix[78]. As shown in Figure 6A, the transmission electron microscopy (TEM) image reveals uniformly cross-linked carbon-coated nanoparticles with an average diameter of approximately 15 nm. The La-doped catalyst exhibited excellent electrocatalytic activity and durability for HER over a wide pH range. The overpotentials of the La-MoP@NC electrode were 129.3 mV in 1.0 M KOH, 142.18 mV in 0.5 M H2SO4, and 235.27 mV in 1.0 M PBS [Figure 6B]. Detailed structural characterizations and DFT calculations confirmed that the La dopants effectively modulated the electronic density around Mo and P atoms, optimizing the Gibbs free energy for hydrogen adsorption on MoP and enhancing its intrinsic HER activity. Subsequently, Ye et al. synthesized La-MoP@N/C with varying La doping ratios, which also showed outstanding HER durability and electrocatalytic performance[79]. The optimized nitrogen-doped carbon encapsulated La-doped MoP (La0.025-Mo0.975P@N/C) achieved a low overpotential of 113 mV at a current density of 10 mA cm-2.
Figure 6. (A) TEM images of La-MoP@NC; (B) The iR-corrected LSV curves of La-MoP@NC in alkaline, acidic, and neutral media. This figure is quoted with permission from Wei et al.[78]; (C) SEM images of the surface of Ho-LaNi5/NF; D: HER polarization curves of Ho-LaNi5/NF, LaNi5/NF, Ni foam, Ni plate and commercial Pt/C. This figure is quoted with permission from Shi et al.[80]; (E) iR-Corrected polarization curves of La-NMS@NF in 1.0 M KOH. This figure is quoted with permission from Jin et al.[82]; (F) The OER and HER properties of HEAs were tested on NF using a three-electrode system; G: Distribution of each element in FeCoNiMnRuLa/CNT under the bright field conditions. This figure is quoted with permission from Zhang et al.[83]; (H) Polarization curves of catalysts for HER. This figure is quoted with permission from Wu et al.[84]. RHE: Reversible hydrogen electrode; NF: nickel foam; NP: nickel plate; CNT: carbon nanotube; HAADF: high-angle annular dark-field; TEM: transmission electron microscopy; SEM: scanning electron microscopy; HER: hydrogen evolution reaction; OER: oxygen evolution reaction; LSV: linear sweep voltammetry; iR: internal resistance; HEA: high-entropy alloy.
Lanthanum (La)-doped nickel-based metal foams combine the structural advantages of NFs with the unique catalytic properties of lanthanum, resulting in enhanced catalytic activity and outstanding thermochemical stability. As shown in Figure 6C, Wu et al. fabricated a hierarchical honeycomb-structured LaNi5 alloy (Ho-LaNi5/NF) on NF[80]. This self-supported electrode exhibits excellent electrical coupling and conductivity between the NF and LaNi5, forming a 3D self-supported heterostructure with remarkable electrocatalytic performance and durability for both the HER and OER. At a high current density of 100 mA cm-2, it achieved an overpotential of 1.86 V, comparable to commercial IrO2//Pt/C coupled electrodes (1.85 V) [Figure 6D]. Additionally, Li et al. synthesized a La-doped Ni3S2/MoS2 heterostructure catalyst (La-NMS@NF) on NF[81]. The incorporation of La dopants induced the formation of nanoflower-like structures with abundant porous channels, which promoted mass transfer and exposure of active sites. In 1.0 M KOH electrolyte, the catalyst achieved a HER overpotential of only 154 mV at 100 mA cm-2 [Figure 6E]. High-entropy effects, lattice distortion, sluggish diffusion, and the cocktail effect collectively endow HEAs with excellent structural stability, abundant active sites, and enhanced intrinsic activity[82]. Wang et al. reported a FeCoNiMnRuLa/CNT HEA catalyst, in which the introduction of La disrupted the lattice order of FeCoNiMnRu, enabling efficient electrocatalytic water splitting[83]. La doping reduced the number of unpaired electrons in FeCoNiMnRu, thereby accelerating the transition from singlet oxygen-containing intermediates to triplet oxygen. During HER, the FeCoNiMnRuLa/CNT catalyst exhibited a low overpotential of 50 mV at 10 mA cm-2 [Figure 6F]. The XRD patterns exhibit distinctive diffraction peaks for multi-walled carbon nanotubes (MWCNTs) at 26.1°, 43.1°, 53.7°, and 78.4°, which remain largely unaltered following the activation process. EDX in bright-field mode confirmed the uniform distribution of all metal elements within the HEA nanoparticles and the successful incorporation of La [Figure 6G]. The catalyst maintained excellent performance even after 200 h of stability testing.
RuO2 tends to form RuO4 and dissolve under acidic water oxidation conditions, which limits its commercial viability due to chemical instability. To address this issue, Wu et al. synthesized La-doped RuO2 nanocrystals through hydrothermal and annealing processes[84]. La doping accelerated the charge transfer rate of electrons or holes, enabling the La-doped RuO2 nanocrystals to exhibit superior electrocatalytic performance in acidic electrolytes. In 0.5 M H2SO4, the catalyst showed a low overpotential of 71 mV at 10 mA cm-2, along with excellent long-term stability. Compared with pure RuO2, its HER overpotential was reduced by 15 mV [Figure 6H]. Rodney et al. synthesized La-doped copper oxide nanoparticles (Cu1-xLaxO) via a co-precipitation method and deposited them on a stainless steel substrate by drop casting[85]. Testing in 1.0 M KOH alkaline electrolyte revealed that 1% La-doped CuO achieved a HER potential of 173 mV at 10 mA cm-2 (vs. RHE).
Cerium-based
Cerium dioxide (CeO2), a rare-earth metal oxide, is widely utilized as a promoter in electrocatalysis due to its abundant oxygen vacancies and the flexible redox transition between Ce3+ and Ce4+. Guo et al. synthesized a CeO2-modified bimetallic phosphide catalyst, (CeO2)-NiCoP[86]. The surface modification by CeO2 effectively regulated the electronic distribution on the bimetallic phosphide, promoting electron transfer from NiCoP to CeO2. This shifted the d-band center of the metal active sites negatively, optimizing the binding strength with hydrogen intermediates and significantly enhancing HER activity. The overpotentials at current densities of 10, 500, and 1000 mA cm−2 were as low as 84, 202, and 242 mV, respectively, which are substantially lower than those of unmodified NiCoP [Figure 7A].
Figure 7. (A) Polarization curves and the corresponding Tafel plots of CeO2-NiCoP, NiCoP and commercial Pt/C catalysts. This figure is quoted with permission from Yu et al.[86]. (B) XRD pattern of Fe2P-CoP/CeO2-20; (C) LSV curves of Fe2P-CoP/CeO2; (D) ΔGH* profiles a on CoP, Fe2P, Fe2P-CoP, and Fe2P-CoP/CeO2. This figure is quoted with permission from Ding et al.[87]; (E) SEM image; (F) Illustration of the HER mechanism on Y, Co-CeO2. This figure is quoted with permission from Liu et al.[89]; (G) HER performance examined in 1.0 M KOH of CeO2-I, CeO2-II, and CeO2-III. This figure is quoted with permission from Liu et al.[90]; (H) DF-TEM image of Ce-doped CoP, and element mapping images of Co, Ce and P, respectively; (I) Polarization curves (with iR corrections) of CoP, Ce-doped CoP and Pt/C catalysts with a scan rate of 5 mV/s in 0.5 M H2SO4. This figure is quoted with permission from Gao et al.[91]. RHE: Reversible hydrogen electrode; XRD: X-ray diffraction; LSV: linear sweep voltammetry; SEM: scanning electron microscopy; LSV: linear sweep voltammetry; iR: internal resistance; DF-TEM: dark-field transmission electron microscopy.
Ding et al. constructed a novel Fe2P-CoP/CeO2 ternary heterojunction via interface engineering and selective phosphorization[87]. After phosphorization under an N2 atmosphere, CoFe-Prussian Blue Analogue-derived/CeO2-20 (CoFe-PBA/CeO2-20) nanocubes transformed into Fe2P-CoP/CeO2 composites. XRD confirmed the successful formation of the ternary heterojunction [Figure 7B]. Electrochemical results demonstrated excellent HER performance for Fe2P-CoP/CeO2-20, with overpotentials of 45 mV at 10 mA cm−2 and 100 mV at 50 mA cm−2 [Figure 7C]. DFT calculations revealed that CeO2 coupling at the Fe2P/CoP interface facilitated electron redistribution across the three-phase boundary, optimized the Gibbs free energy for H* adsorption, and significantly reduced the energy barrier for water dissociation, thereby enhancing alkaline water splitting [Figure 7D].
Guo et al. designed a CeO2@CoSe2 nano-needle catalyst supported on carbon cloth (CeO2@CoSe2/CC) via interface engineering[88]. The catalyst exhibited outstanding HER performance, attributed to the synergistic effects of CeO2 in promoting H2O dissociation and CoSe2 in facilitating H* adsorption. Additionally, Liu et al. developed a Y and Co co-doped cerium dioxide (Y, Co-CeO2) catalyst to improve the performance of CeO2 in alkaline HER[89]. As shown in the SEM image [Figure 7E], the catalyst exhibited a nanosheet morphology. Lattice compression in CeO2 increased oxygen vacancy concentration, which activated neighboring sites for hydrogen adsorption and significantly improved HER kinetics. The catalyst achieved an ultra-low overpotential of only 27 mV at 10 mA cm-2, surpassing even Pt-based electrocatalysts [Figure 7F].
Recent studies have increasingly focused on two-dimensional (2D) structures due to their high surface exposure and compatibility with frustrated Lewis pair (FLP) active site designs. Liu et al. synthesized 2D CeO2 nanosheets with varying oxygen vacancy concentrations and found that those with the highest oxygen vacancy concentration showed the best HER performance, achieving an overpotential of 132 mV at 10 mA cm-2 [Figure 7G][90]. This work provides a theoretical and technical foundation for designing advanced FLP-type HER catalysts.
Cerium can also be doped into host catalysts to enhance HER performance. Gao et al. combined theoretical and experimental approaches to demonstrate that doping Ce into CoP adjusts the electronic structure and lowers the hydrogen adsorption free energy[91]. Dark-field TEM and EDS mapping [Figure 7H] showed that Ce is not only stably incorporated but also uniformly distributed with Co and P, confirming successful doping without disrupting the CoP matrix. Compared to undoped CoP, Ce-doped CoP exhibited lower overpotentials, Tafel slopes, and charge transfer resistance, along with higher electrochemical surface area and turnover frequency, resulting in excellent catalytic activity and stability. Notably, in both acidic and alkaline media, the optimized Ce-doped CoP catalyst exhibited ultra-low overpotentials of 54 mV and 92 mV, respectively, at 10 mA cm−2 [Figure 7I]. Finally, Zhang et al. synthesized a Ce-doped NiCoP catalyst (Ce-NiCoP) supported on nickel foam via a simple solvothermal method[92]. The presence of Ce promoted the in situ formation of a hydrophilic NiCoP phase, resulting in outstanding HER performance.
Erbium-based
Erbium (Er), a REE with a unique 4f electronic configuration, can generate oxygen vacancies and lattice defects when doped into oxide structures. These defects provide additional active sites for electrocatalytic reactions, thereby enhancing both catalytic activity and stability, especially under acidic conditions for the HER[93]. As shown in Figure 8A, Zhang et al. synthesized Er-doped CoP nanomeshes that exhibited larger specific surface areas and higher double-layer capacitance, indicating more exposed active sites[94]. This enhancement was attributed to electronic structure modulation, particularly the optimization of the d-band center of Co sites, which brought the adsorption free energy of hydrogen intermediates (ΔGH*) closer to the ideal value. As a result, the Er-doped CoP nanomesh demonstrated excellent HER performance in both acidic and alkaline media, with overpotentials of just 52 mV and 66 mV at 10 mA cm-2, respectively, and maintained stability over 25 h [Figure 8B]. XPS analysis confirmed the successful incorporation of Er into the CoP lattice, evidenced by the appearance of a new peak at 171 eV in the Er-doped sample compared to pristine CoP [Figure 8C]. Additionally, Zhang et al. prepared vertically aligned Er-doped NiCoP nanowire arrays (Er-NiCoP/NF) grown on conductive NF[95]. These electrodes displayed remarkable bifunctional catalytic activity, with an HER overpotential of only 46 mV at 10 mA cm-2 [Figure 8D]. XPS analysis revealed a new Er 4d orbital peak at 168.8 eV [Figure 8E], confirming Er incorporation. DFT calculations further showed that Er doping adjusted the electronic structure of NiCoP, lowering the d-band center of Ni and Co atoms relative to the Fermi level. This shift optimized the Gibbs free energy for HER intermediates, thereby accelerating water-splitting kinetics [Figure 8F].
Figure 8. (A) TEM images of the Er-doped CoP nanomesh; (B) Polarization curves of bare CC, CoP, Er-doped CoP and Pt/C in 1.0 M KOH; (C) XPS full spectra of CoP and Er-doped CoP, respectively. This figure is quoted with permission from Zhang et al.[94]; (D) LSV polarization curves of Er-NiCoP/NF, NiCoP/NF, bare NF, and commercial Pt/C for HER; (E) Calculated free-energy diagram of NiCoP and Er-NiCoP for HER; (F) XPS analysis of survey scan and high resolution. This figure is quoted with permission from Zhang et al.[95]. (G) Aberration-corrected (AC) HAADF-STEM image of Pt-Er/h-NC; (H) Polarization curves of Pt-Er/h-NC and the references; (I) The theoretical volcano of HER on Pt atoms of various Pt-based catalysts. This figure is quoted with permission from Chen et al.[97]. RHE: Reversible hydrogen electrode; TEM: transmission electron microscopy; XPS: X-ray photoelectron spectroscopy; LSV: linear sweep voltammetry; NF: nickel foam; HER: hydrogen evolution reaction; HAADF-STEM: high-angle annular dark-field scanning transmission electron microscopy; NC: nitrogen-doped carbon.
Nadeem et al. synthesized a bimetallic Pt-Er composite (Pt-Er@PCN920) by thermally decomposing a Zn-based metal-organic framework (Zn-MOF) under an inert atmosphere to obtain a nitrogen-doped porous carbon support[96]. The resulting material possessed a porous structure and high surface area, enabling efficient HER activity[96]. Chen et al. developed a dual-function catalyst (Pt-Er/h-NC) featuring Pt-Er alloy clusters, atomically dispersed Pt, and single Er atoms [Figure 8G][97]. This nanoarchitecture introduced abundant heterojunctions and boundary sites, providing more active centers for HER. Pt-Er/h-NC achieved an ultra-low overpotential of 25 mV at 10 mA cm-2 in acidic media [Figure 8H]. The synergy among the nanostructured active sites, combined with Er-induced stabilization, significantly improved catalytic performance. DFT calculations [Figure 8I] revealed that Er modulates the hydrogen adsorption energy at Pt sites toward ~ 0 eV, resulting in higher current densities compared to pure Pt catalysts under the same conditions. Fatima et al. fabricated a highly efficient bifunctional catalyst by ultrasonically anchoring Er onto V2CTx MXene nanosheets, forming Er@V2CTx nanocomposites[98]. At an optimal Er-to-V2CTx mass ratio of 0.12:1, the composite exhibited excellent HER performance in 1.0 M KOH, with an overpotential of 174 mV[98].
Other rare earth-based catalysts
Beyond La, Ce, and Er, other REEs have also been explored for enhancing the performance of HER electrocatalysts. Xu et al. investigated the HER activity of Ba0.5Sr0.5Co0.8Fe0.2O3-σ (BSCF), a widely studied perovskite oxide in electrocatalysis[99]. As shown in Figure 9A, its general formula ABO3 (where A is a rare earth or an alkali; B is a transition metal; and O is oxygen) allows for rare-earth or alkali metals at the A-site and TMs at the B-site. Through simple A-site doping with praseodymium (Pr), the surface electronic structure and catalytic performance were effectively modified. The resulting Pr0.5Ba0.5Sr0.5Co0.8Fe0.2O3-σ (Pr0.5BSCF) demonstrated enhanced HER activity and durability in alkaline media, requiring an overpotential of only 237 mV at 10 mA cm-2, more than 100 mV lower than undoped BSCF (342 mV) [Figure 9B], with a Tafel slope of just 45 mV dec-1. Raman spectroscopy confirmed that Pr0.5BSCF maintained a cubic perovskite structure with Pm-3m symmetry [Figure 9C]. Moreover, the Pr0.5BSCF-based electrode sustained stable hydrogen production at a high current density of 50 mA cm-2 for 25 h, outperforming many other bulk or nanoscale non-Pt HER catalysts under strong alkaline conditions[99]. Subsequently, Liu et al. developed a novel Ni-Ce-Pr-Ho electrocatalyst using a one-step constant-current electrodeposition method [Figure 9D][100]. Characterization and electrochemical tests revealed that this multicomponent system displayed excellent HER activity in 1 M KOH, achieving an overpotential as low as 78 mV at 10 mA cm-2.
Figure 9. (A) The structure of ABO3 perovskite oxide (where A is a rare earth or an alkali, blue; B is a transition metal, olive; and O is oxygen, red); (B) Polarization curves of BSCF, Pr0.5BSCF and commercial Pt/C catalysts. The background HER activity of a conductive-carbon-supported GC electrode is shown for reference; (C) Raman spectra of BSCF and Pr0.5BSCF. This figure is quoted with permission from Xu et al.[99]; (D) Schematic illustration of the one-step electrodeposition for Ni-Ce-Pr-Ho catalyst. This figure is quoted with permission from Liu et al.[100]; (E) HER LSV curves of various electrocatalysts. This figure is quoted with permission from Xu et al.[101]; (F) Schematic of the AEMWE; G: Polarization curve in AEMWE. This figure is quoted with permission from Liu et al.[102]. RHE: Reversible hydrogen electrode; NF: nickel foam; BSCF: Ba0.5Sr0.5Co0.8Fe0.2O3-σ; HER: hydrogen evolution reaction; LSV: linear sweep voltammetry; AEMWE: anion exchange membrane water electrolysis.
Wang et al. reported a bifunctional “armored” catalyst consisting of NiGd nanoparticles encapsulated within a nitrogen-doped carbon shell (NiGd@N-C). Owing to its distinctive core-shell architecture, this catalyst exhibited outstanding HER performance in alkaline media, with an overpotential of only 45 mV at 10 mA cm-2 [Figure 9E] and remarkable stability over 60 h. This was significantly better than the Gd-free counterpart (112 mV) and comparable to commercial Pt/C (26 mV). DFT calculations showed that the introduction of Gd, which has lower electronegativity than Ni, promoted electron penetration from the NiGd alloy core to the N-C shell, leading to interfacial charge redistribution and electron accumulation on the N-C shell surface. This optimized electronic configuration significantly reduced the ΔGH* to near-zero values, lowering the reaction energy barrier, accelerating HER kinetics, and enhancing charge transfer. The synergy between the physical protection of the core-shell structure and the Gd-induced electronic effects is key to the high activity and long-term stability of the NiGd@N-C catalyst[101].
To bridge the gap between laboratory-scale demonstrations and industrial deployment, incorporating REEs into electrocatalysts must prioritize scalability, cost-effectiveness, and compatibility with real-world electrolyzer systems. A key challenge lies in translating the enhanced activity and stability observed in small-scale tests to large-area electrodes operating under high current densities, where mass transport limitations, ohmic losses, and thermal management become pronounced. For practical implementation, REE-based catalysts should be evaluated in full-cell configurations, such as anion exchange membrane water electrolysis (AEMWE) and proton exchange membrane water electrolysis (PEMWE), which are pivotal for industrial hydrogen production due to their high efficiency and compact design. Liu et al. demonstrated the industrial relevance of Nd2O3@Ru heterojunctions supported on carbon nanotubes (Nd2O3@Ru/CNT) as a cathode catalyst in an AEMWE setup for overall water splitting[102]. The AEMWE device, assembled with Nd2O3@Ru/CNT as the cathode, commercial RuO2 as the anode, and a Fuma FAA-PK-130 anion exchange membrane [Figure 9F], also achieved 1.494 V at 100 mA cm-2 and maintained operational stability for over 100 h at this current density without significant degradation. This REE-engineered material exhibited exceptional performance, requiring cell voltages of only 1.665 V and 1.806 V to achieve high current densities of 500 mA cm-2 and 1,000 mA cm-2 [Figure 9G], respectively, in 1 M KOH electrolyte. The superior performance and durability arise from the asymmetrical neodymium (Nd)-oxygen (O)-Ru bridge, where oxygen’s p-orbital mediates f-d orbital coupling between Nd and Ru, optimizing water adsorption, hydrogen evolution kinetics, and resistance to nanoparticle agglomeration under high-current conditions, as supported by in situ Raman spectroscopy and DFT calculations. This example highlights the potential of REE heterostructures to enable AEMWE operations at industrially relevant high current densities, promoting efficient and scalable green hydrogen production while reducing reliance on precious metals such as Pt. Extending such orbital coupling strategies to PEMWE systems could further harness the versatility of REEs across acidic and alkaline environments, facilitating broader commercialization.
In summary, rare earth-based catalysts exhibit diverse structural features and electronic properties depending on the specific element incorporated, host material, and synthetic strategy. Across La-, Ce-, Er-, and other rare-earth systems, these catalysts demonstrate varying degrees of enhancement in HER activity, stability, and intermediate adsorption behavior. Representative catalysts are systematically summarized in Table 1. An intuitive comparative illustration is further presented in Figure 10.
Figure 10. Comparison of HER electrocatalytic performance across different REE types. NC: Nitrogen-doped carbon; N/C: CNT: carbon nanotube; BSCF: Ba0.5Sr0.5Co0.8Fe0.2O3-σ; NF: nickel foam; HER: hydrogen evolution reaction; REE: rare earth element.
Summary of various REE-type HER electrocatalysts and their HER performance
| Catalysts | REE type | Electrolytes | Overpotential (mV)(η10) | Stability(h) | Ref. |
| La-MoP@NC | La | 1.0M KOH | 129.3 (iR) | 12 | [78] |
| La0.025Mo0.975P@N/C | La | 1.0M KOH | 113 (90%iR) | 24 | [79] |
| Ho-LaNi5/NF | La | 1.0M KOH | 136 | 10 | [80] |
| FeCoNiMnRuLa/CNT | La | 1.0M KOH | 50 | 120 | [83] |
| La-RuO2 | La | 0.5M H2SO4 | 71 (95%iR) | 28 | [84] |
| (CeO2)-NiCoP | Ce | 1.0M KOH | 84 (85%iR) | 18 | [86] |
| Fe2P-CoP/CeO2-20 | Ce | 1.0M KOH | 45 (iR) | 30 | [87] |
| Y,Co-CeO2 | Ce | 1.0M KOH | 27 | 200 | [89] |
| Ce-CoP | Ce | 1.0M KOH | 92 (iR) | 10 | [91] |
| 0.5 M H2SO4 | 54 | 10 | |||
| Er-CoP | Er | 1.0M KOH | 84 | 20 | [94] |
| Er-NiCoP/NF | Er | 1.0M KOH | 46 (90%iR) | 48 | [95] |
| Pt-Er/h-NC | Er | 0.5 M H2SO4 | 25 | 130 | [97] |
| Pr0.5BSCF | Pr | 1.0M KOH | 237 | 25 | [99] |
| NiGd@N-C | Gd | 1.0M KOH | 45 (95%iR) | 60 | [101] |
| Nd2O3@Ru/CNT | Nd | 1.0M KOH | 260 (η500) | 100 | [102] |
APPLICATION OF RARE EARTH-BASED CATALYSTS IN OER
In the HER, REEs primarily function as electronic regulators. Due to their low electronegativity and strong electropositivity, RE doping can donate electrons to adjacent TM centers, modulating their d-band center positions. This optimization of the hydrogen adsorption free energy (ΔGH*) facilitates water molecule dissociation (Volmer step) and hydrogen atom recombination (Heyrovsky/Tafel steps), thereby enhancing the kinetic performance of hydrogen evolution. In the OER, the regulatory role of REEs is more complex. RE elements typically serve as oxygen-affinitive activators, capable of stabilizing high-valence intermediates. Additionally, through ionic radius mismatch, they induce lattice strain, generating oxygen vacancies that promote oxygen ion migration and lattice oxygen participation in the reaction. Building on the design principles established for HER, these effects allow REEs to finely tune the electronic structure and geometric environment of OER catalysts, which involve multiple proton-electron coupled steps and oxygenated intermediates. In the following sections, we classify representative OER catalysts according to the specific REE employed (La, Ce, Er, and other rare-earths) highlighting their distinct electronic and geometric modulation effects, mechanistic insights, and performance characteristics.
Lanthanum-based
Compared to HER, OER is a more complex four-electron transfer process. Its reaction pathway involves four key intermediates: OH*, O*, OOH*, and O2*, encompassing multiple consecutive elementary steps[103,104]. Lanthanum (La)-doped nickel-based catalysts enhance OER efficiency and stability by tuning the d-band center of Ni, thereby optimizing the interaction between the catalyst and oxygenated intermediates, and lowering the reaction energy barriers. This doping strategy not only improves catalytic activity but also significantly enhances durability, offering a novel approach to designing high-performance OER catalysts. Wang et al. reported a ternary NiFeLa catalyst containing unique M-NiFe (M: 4d-Mo/5d-La) units with distinct d-orbitals[105]. Both experimental results and theoretical calculations demonstrated that La doping optimized the hybridization between the d-orbitals of NiFeLa and the O-2p orbitals, enhancing the adsorption strength toward oxygen intermediates and reducing the energy barrier of the rate-determining step, thus boosting OER performance. The synthesized NiFeLa catalyst required only 1.58 V overpotential to achieve a high current density of 1,000 mA cm-2 in an anion exchange membrane electrolyzer, maintaining excellent long-term stability over 600 h. Furthermore, Arumugam et al. investigated the influence of La doping on the catalytic activity and durability of CoMoO4 in high pH media for overall water splitting[106]. By doping La at various concentrations into the CoMoO4 lattice, the electrocatalytic performance was regulated. The optimal performance was achieved with 5% La-doped CoMoO4, which exhibited a low overpotential of 272 mV at 20 mA cm-2 [Figure 11A]. Notably, La doping stabilized the lattice structure, optimized electronic distribution, and accelerated surface reconstruction into a stable γ-CoOOH phase, mitigating the severe MoO42- leaching issue in the electrolyte and preserving structural integrity. DFT calculations revealed that La doping altered the electronic structure of CoMoO4, effectively optimizing the adsorption energies of reactive hydrogen and oxygen intermediates and enhancing the intrinsic OER activity of CoMoO4 [Figure 11B]. Song et al. also fabricated a La-doped nickel-iron layered double hydroxide/nickel sulfide heterostructure (La-NiFeLDH/NiS/NF) on nickel foam using a solution-based chemical etching method followed by one-step electrodeposition[107]. As shown in Figure 11C, XPS confirmed the incorporation of La into NiFeLDH. This electrocatalyst demonstrated outstanding OER performance, requiring only 290 mV to reach 1,000 mA cm-2 [Figure 11D], with remarkable durability over 200 h. Both theoretical and experimental results indicated the formation of strong Ni-S(O)-Ni(Fe) coupling interfaces within the heterostructure. DFT calculation results further showed that La doping facilitated electron transfer from NiS to NiFeLDH, tuned the d-band center of Fe atoms, optimized the binding strength to oxygen intermediates, and lowered the activation energy of the reaction [Figure 11E]. In addition, Nguyen et al. reported a novel La-based high-entropy perovskite oxide (HEPO) electrocatalyst for OER[108]. The B-site of the HEPO lattice was composed of five successive 3d TMs: Cr, Mn, Fe, Co, and Ni. In the five non-equimolar HEPO samples, one TM in each sample had double the concentration of the others. All HEPOs outperformed conventional single-component perovskite oxides. The optimized La(CrMnFeCo2Ni)O3 HEPO delivered a low overpotential of 325 mV at 10 mA cm-2 and exhibited excellent electrochemical stability over 50 h.
Figure 11. (A) LSV responses of different La-doped CoMoO4 catalysts toward the OER in 1 M KOH; (B) Free energy plots of OER intermediates on the Co-site in the 5% La-CoMoO4 system under different thermodynamic potentials in alkaline media. This figure is quoted with permission from Arumugam et al.[106]; (C) XPS spectrum of La-NiFe LDH/NiS/NF La 3d; (D) Polarization curves of different electrodes; (E) Free energy diagram. This figure is quoted with permission from Song et al.[107]; (F) LSV curves at 10 mA cm-2 and current densities at 1.6 V of commercial RuO2, RuO2, and LaxRu1-xO2; (G) Reaction free energy of the acidic OER on RuO2 and La0.1Ru0.9O2. This figure is quoted with permission from Li et al.[111]; (H) XPS spectra of O 1s for La3IrO7 and La3IrO7-SLD; (I) Polarization curves of La3IrO7-SLD and commercial IrO2 at a scan rate of 5 mV s-1. This figure is quoted with permission from Chen et al.[112]; (J) Schematic of the PEM electrolyzer device and I-V curves of PEM electrolyzer using La-RuO2. This figure is quoted with permission from Zhang et al.[113]. RHE: Reversible hydrogen electrode; NF: nickel foam; LSV: linear sweep voltammetry; OER: oxygen evolution reaction; XPS: X-ray photoelectron spectroscopy; PEM: proton exchange membrane; SLD: surface La-defective.
Hao et al. designed ultrasmall (2 nm average diameter) Pt and La co-doped IrO2 nanoparticles (Pt0.1La0.1IrO2@NC) highly dispersed on nitrogen-doped carbon (NC)[109]. In a 0.5 M H2SO4 solution, Pt0.1La0.1IrO2@NC exhibited a record-low OER overpotential of only 205 mV at 10 mA cm-2, along with excellent long-term stability of 135 h. Theoretical calculations indicated that co-doping Pt and La into the IrO2 lattice effectively tuned the d-band center and lowered the energy barrier of the rate-determining step (O to OOH*) in the OER pathway. Gan et al. synthesized a La and sulfur co-doped FeOOH/NiOOH multiphase electrocatalyst[110]. FeOOH in the system reduced the formation energy of NiOOH and enhanced its stability as an active HER site. The formed abundant oxygen vacancies increased the number of active sites, optimized intermediate adsorption, and improved conductivity. Moreover, La and S co-doping modulated the electronic structure of FeOOH. As a result, the resulting catalyst exhibited low overpotentials of 210/450 mV at 100/1,000 mA cm-2, a small Tafel slope (32 mV dec-1), and outstanding stability up to 60 h at 1,000 mA cm-2. Additionally, in 30 wt% KOH at 60 °C, the catalyst achieved excellent OER performance with only 180 mV overpotential at 250 mA cm-2, maintaining catalytic durability for 135 h at this current density.
Li et al. developed a facile sol-gel method to construct asymmetric Ru-O-La structures within RuO2, which modulate electron redistribution and suppress Ru over-oxidation[111]. In acidic media, the optimal La0.1Ru0.9O2 catalyst required only 188 mV to achieve 10 mA cm-2 [Figure 11F] and exhibited prolonged stability of 63 h at 1.6 V vs. RHE, far surpassing the 8 h stability of standard RuO2. Combined experimental and theoretical analyses revealed that the Ru-O-La asymmetric structure facilitated electron transfer from La to Ru, increasing the electron density around Ru and preventing over-oxidation. Moreover, this electron redistribution shifted the Ru 4d-band center, thereby optimizing the adsorption and desorption of oxygen intermediates [Figure 11G]. Qin et al. also reported a surface La-defective IrOx catalyst (La3IrO7-SLD) prepared via electrochemical activation, which outperformed commercial IrO2 and most reported Ir-based catalysts in both OER activity and durability[112]. As shown in Figure 11H, comparisons of XPS spectra before and after activation revealed a significant increase in surface hydroxylation, correlating with La leaching. After OER testing, the main O 1s XPS peak at 531.6 eV (assigned to Ir-OH species) broadened notably, indicating a surface reconstruction with a high hydroxyl content (71.7% vs. 34.9% in pristine La3IrO7), thus exposing more Ir active sites. Meanwhile, the La 3d and 4d peaks were significantly reduced post-OER, confirming La leaching and formation of La-deficient structures. As shown in Figure 11I, La3IrO7-SLD achieved 10 mA cm-2 at an overpotential of only 296 mV, outperforming IrO2 (316 mV). DFT calculations revealed that the Ir-centered lattice oxygen mechanism (LOM) endowed La3IrO7-SLD with superior catalytic activity and stability, as the Ir 5d orbitals are positioned at a more favorable energy level relative to the O 2p orbitals. In contrast, the free energy barrier for *OOH formation via the adsorbate evolution mechanism (AEM) pathway reaches 2.73 eV, which is significantly higher than those of IrO2(110) and La3IrO7 (001). This result is inconsistent with the electrochemical observations, suggesting that La3IrO7-SLD is more likely to follow a lattice-oxygen-involved reaction pathway.
As a compelling case study, Zhang et al. demonstrated the industrial relevance of La-doped RuO2 nanorods grown in situ on titanium mesh (La-RuO2@TM) as an anode catalyst in a PEMWE setup[113]. This REE-engineered material exhibited exceptional performance, requiring a cell voltage of only 1.815 V to achieve a high current density of 1 A cm-2, while maintaining operational stability for over 120 h at 60 °C without significant degradation. Notably, this outperformed both undoped RuO2@TM and commercial IrO2 benchmarks under identical conditions, with lower voltages also recorded at 0.5 A cm-2 (1.692 V) and 2 A cm-2 (2.051 V). The enhanced durability stems from La doping, which forms a La-O-Ru local structure that modulates intermediate adsorption, mitigates Ru leaching, and reduces lattice oxygen loss, as corroborated by DFT calculations. This example underscores the potential of REE doping to enable PEMWE operations at industrially relevant high current densities, facilitating efficient green hydrogen production at scale. Extending such strategies to AEMWE systems could further leverage the electronic tunability of REEs in alkaline environments, potentially reducing reliance on precious metals and lowering overall system costs for widespread commercialization.
Despite the progress of La doping strategies in reducing reliance on noble metals (e.g., Ir-based systems) and improving performance at industrial-level current densities, large-scale application remains constrained by complex synthesis procedures (e.g., multicomponent precursor control in HEAs, precision interface engineering in heterostructures) and unclear dynamic stability mechanisms (e.g., oxygen vacancy evolution, surface reconstruction). Future research should focus on developing non-noble metal La-based high-entropy systems, employing in-situ characterization techniques to unravel dynamic active site evolution, and exploring green synthesis routes to promote practical application of La-doped catalysts in water electrolytic oxygen production.
Cerium-based
Ce-based catalysts can enhance their OER activity through strategies such as doping, interface engineering, and the construction of heterostructures. Wang et al. reported a mechanism based on interfacial electronic redistribution, in which electron transfer from Ce to Ni and Co in the CeO2/NiCo2S4 heterostructure leads to the stabilization of metal-sulfur (M-S) bonds[114]. This effect effectively suppresses sulfur leaching during OER. As shown in Figure 12A, the XRD pattern of CeO2/NiCo2S4 corresponds well with the cubic phase CeO2, respectively, with no impurity peaks observed. This confirms the successful formation of the heterostructure without chemical reactions between phases. Moreover, the well-modulated interface enhances the adsorption affinity toward oxygen intermediates and reduces the energy barrier for OER. Consequently, the CeO2/NiCo2S4 catalyst exhibits outstanding OER performance, delivering ultralow overpotentials of only 146 and 271 mV at 10 and 100 mA cm-2, respectively [Figure 12B]. More importantly, it demonstrates exceptional durability, maintaining stable performance at 500 mA cm-2 for over 200 h - surpassing pristine NiCo2S4 and most reported TMC-based catalysts. Zhi et al. also proposed a new strategy, introducing a CeO2 layer onto S-Co(OH)2 to stabilize catalysts operating via the LOM[115]. Explicit experimental identification of the switched reaction pathway to LOM in S-Co(OH)2/CeO2 has been achieved through pH-dependent OER activity measurements and tetramethylammonium cation (TMA) probe experiments. In situ XPS, Raman spectroscopy, and other studies indicate that CeO2 acts as an electron buffer during OER, either accepting or releasing electrons at different stages. Specifically, at the initial voltage, CeO2 extracts electrons from Co(OH)2, promoting the generation of high-valence Coδ+ species, thereby accelerating surface reconstruction and forming active CoOOH. With increasing potential, the reconstructed CoOOH evolves into CoO2 with higher work function, which then extracts electrons from the CeO2 “reservoir” rather than from M-O bonds, ensuring stability throughout the OER process. Additionally, the introduced S atoms enhance Co-O covalency and trigger the LOM pathway. As a result, the S-Co(OH)2/CeO2 hybrid delivers excellent OER performance in 1.0 M KOH. The inter requires only 227 mV to achieve 10 mA cm-2, and operates stably over 100 h at 1000 mA cm-2 in a self-assembled anion exchange membrane electrolyzer, demonstrating the pivotal role of the electron reservoir concept in the development of sustainable LOM-based electrocatalysts. Song et al. constructed a RuO2/CeO2 heterostructured catalyst via lattice-matched Ru-O-Ce bridges, which can effectively facilitate electron transfer between Ru and Ce species, and further induce lattice strain that distorts the local crystalline structure of RuO2[116]. This catalyst demonstrates remarkable stability, with negligible performance degradation after 1,000 h of continuous OER operation in 0.5 M H2SO4, while maintaining high activity with an overpotential of only 180 mV at 10 mA cm-2. In situ attenuated total reflection-surface enhanced infrared absorption spectroscopy (ATR-SEIRAS), differential electrochemical mass spectrometry (DEMS), and DFT calculations reveal that RuO2 sites at the interface and non-interface promote the oxide path mechanism (OPM) and the enhanced AEM, respectively. You et al. constructed a 0D/2D heterostructure by decorating SrIrO3 nanosheets with CeO2 quantum dots, aiming for durable and efficient OER in strongly acidic conditions[22]. The fast Fourier transform (FFT) image [Figure 12C] confirms the formation of the CeO2/SrIrO3 heterostructure. Theoretical calculations reveal that CeO2 with electron rearrangement not only reduces the OER energy barrier but also mitigates Sr leaching. Benefiting from the dual role of CeO2 in activation and stabilization, the CeO2@SrIrO3 heterostructure delivers excellent performance with an overpotential of only 238 mV to achieve 10 mA cm-2, maintaining operation for over 50 h, outperforming most perovskite-based electrocatalysts [Figure 12D].
Figure 12. (A) XRD images of CeO2/NiCo2S4; (B) LSV curves of as-prepared catalysts. This figure is quoted with permission from Wang et al.[114]; (C) FFT pattern of 4CeO2@SrIrO3; (D) LSV curve of the commercial IrO2, SrIrO3, 2CeO2@SrIrO3, 4CeO2@SrIrO3 and 8CeO2@SrIrO3 electrocatalysts. This figure is quoted with permission from You et al.[22]; (E) HRTEM image, the marked area (orange line) presents the defects; (F) LSV curves of RuO2 and the prepared ZIF-67@Co(OH)2, Co@Co(OH)2, Ce-Co(OH)2 and Ce0.21@Co(OH)2 in 1.0 M KOH. This figure is quoted with permission from Zhou et al.[118]; (G) Mo 3d spectra for Ru3MoOx and Ru3MoCeOx; (H) Ce3d spectra for Ru3CeOx and Ru3MoCeOx. Erbium-based; (I) LSV curves for RuO2, Ru3CeOx, Ru3MoOx, Ru3MoCeOx, and Ru3MoCe2Ox. This figure is quoted with permission from He et al.[120]. RHE: Reversible hydrogen electrode; XRD: X-ray diffraction; LSV: linear sweep voltammetry; FFT: fast Fourier transform; HRTEM: high-resolution transmission electron microscopy; ZIF: zeolitic imidazolate framework.
Ce-doping could shift the reaction mechanism from the conventional AEM to the LOM, thereby lowering the overall reaction barrier[117]. Zhou et al. synthesized Ce0.21-Co(OH)2 to facilitate rapid electron transfer and improve OER activity and stability[118]. As shown in Figure 12E, high-resolution TEM (HRTEM) images reveal a lattice spacing of 0.314 nm matching the (111) plane of CeO2, indicating the embedding of CeOx into the Co(OH)2 matrix. The core-shell structure features vertically aligned porous Co(OH)2 nanosheets that expose abundant active sites while enabling efficient Ce doping. Utilizing a high-pressure microwave-assisted technique, Ce was homogeneously incorporated into the matrix, forming tightly bound Cex-Co(OH)2 with excellent phase dispersion. The interaction between Ce and Co species greatly accelerates surface electron transfer. Additionally, Ce promotes the formation of Co-superoxide intermediates and facilitates O2 desorption, both of which are crucial for OER rate determination. Linear sweep voltammetry (LSV) curves show an overpotential of only 300 mV at 10 mA cm-2 [Figure 12F]. He et al. developed Ce-NiO@MoO2 catalysts using MoO2 nanorods as supports[119]. The introduction of Ce triggered the formation of flower-like NiO on the MoO2 surface. Ce doping induced the generation of abundant high-valence, unsaturated Ni coordination sites as active centers, while tuning the Ni 3d band center upward, thereby enhancing the adsorption of oxygen intermediates and significantly lowering the energy barrier of the rate-determining OH* → O* step. As a result, Ce-NiO@MoO2Ni3 catalysts exhibited excellent OER performance, operating stably at 100 mA cm-2 with a low overpotential of only 313 mV and sustaining over 200 h of continuous operation. This performance is attributed to the abundance of unsaturated Ni active sites and the highly ordered hierarchical structure that facilitates charge and mass transport. He et al. co-doped RuO2 with Mo and Ce, both featuring relatively large ionic radii, to modulate the local electronic structure of Ru-O bonds, thereby simultaneously enhancing catalytic activity and stability. XPS analysis confirms the successful incorporation of Mo and Ce into RuO2 [Figure 12G and H][120]. The resulting Ru3MoCeOx catalyst shows outstanding activity, with an overpotential of only 164 mV at 10 mA cm-2 and stable performance in acidic medium for over 100 h at 100 mA cm-2 [Figure 12I]. DFT and microscopic analysis reveal that Mo and Ce dopants effectively modulate the covalency of Ru-O bonds, tune electronic correlations, and lower the reaction barrier from 0.78 eV to 0.60 eV. These features make Ru3MoCeOx a promising anode catalyst for PEM electrolyzers. Ni-Fe layered double hydroxides (NiFe-LDHs) are widely regarded as the most active OER catalysts in alkaline media due to their distinctive layered structure and physicochemical properties. Liao et al. proposed a doping strategy to introduce Ce into NiFe-LDH on carbon paper (NiFeCe-LDH@CP), inducing structural disorder and unique lattice distortion[121]. This distortion increases accessible surface area and generates more oxygen vacancies, accelerating OER via electronic structure modulation and intermediate adsorption optimization. The optimized NiFeCe-LDH@CP exhibits excellent durability over 70 h and achieves a high current density of 100 mA cm-2 at a low overpotential of only 267 mV, 41 mV lower than that of pristine NiFe-LDH@CP. Theoretical calculations suggest that lattice distortion modulates the electronic structure of Ni at the active sites and lowers the energy barrier, thus significantly enhancing OER activity.
Erbium-based
Pan et al. proposed a strategy to synergistically enhance catalytic activity through erbium (Er) doping, aiming to improve the intrinsic OER activity and stability of Co3O4[122]. As shown in Figure 13A, the Co3+/Co2+ ratio in 4% Er-doped Co3O4 (Er-Co3O4) reaches 0.97, significantly higher than the 0.59 observed in undoped Co3O4, which positively correlates with OER activity in acidic media. This increase in Co3+ content can be attributed to Er doping, which induces structural modification and promotes electronic interactions between Co and Er. The low-cost Er-Co3O4 catalyst with 4% Er exhibits notably enhanced performance, delivering an overpotential of 321 ± 5 mV at 10 mA cm-2 [Figure 13B], along with excellent long-term stability exceeding 250 h. Moreover, Er incorporation into the spinel Co3O4 structure introduces structural defects and oxygen vacancies, and markedly elevates the Co3+/Co2+ ratio. The catalytic activity of this material is strongly influenced by the ratio of octahedrally coordinated Co3+ and tetrahedrally coordinated Co2+ cations, which constitute the spinel structure. Microkinetic modeling and theoretical calculations further confirm that Er-Co3O4 achieves a significantly optimized Gibbs free energy difference (ΔGO*-ΔGOH*), bringing its OER activity at target current densities close to the theoretical maximum [Figure 13C]. In addition, Zhu et al. enhanced the OER performance of layered double hydroxides (LDHs) by constructing Er-doped nickel-iron LDH catalysts supported on nickel foam (Er-NiFe-LDH@NF)[123]. As illustrated in Figure 13D and E, operando Raman spectroscopy revealed that OH- adsorption at Ni sites was markedly enhanced in Er-NiFe-LDH prior to 450 mV, compared to the undoped catalyst. This is evidenced by the increase in Raman signal intensity, indicating that Er doping facilitated the transformation from Ni-OH to Ni-OOH, thereby accelerating OER kinetics. The optimized Er-NiFe-LDH@NF demonstrated a remarkably low overpotential of just 191 mV to achieve 10 mA cm-2 [Figure 13F], outperforming its undoped counterpart. Theoretical calculations suggest that Er incorporation optimizes the d-band center of NiFe-LDH, promotes spin crossover of valence electrons, and brings ΔG*O-ΔG*OH closer to the peak of the OER volcano plot. This balances the binding strengths of oxygen and hydroxyl intermediates, resulting in superior catalytic performance.
Figure 13. (A) Ratio of Co2⁺: Co3⁺ in Co3O4 and 4% Er-Co3O4; (B) LSV curves of different catalysts at 1 mV/s scan rate and negative scan after iR correction; (C) Microkinetic OER activity volcano model at 10 mA cm-2 as a function of GO-GHO. This figure is quoted with permission from Pan et al.[122]; (D) The in situ Raman spectra of NiFe-LDH@NF and (E) Er-NiFe-LDH@NF for OER in 0.1 M KOH solution; (F) Comparison of the OER activity of catalysts’ polarization curves in 1.0 M KOH solution. This figure is quoted with permission from Zhu et al.[123]. RHE: Reversible hydrogen electrode; LSV: linear sweep voltammetry; iR: internal resistance; OER: oxygen evolution reaction; GO-GHO: the difference between the Gibbs free energy of O* and OH*; LDH: layered double hydroxide; NF: nickel foam.
Recent progress in REE-doped electrocatalysts for the OER has primarily focused on modulating electronic structures to optimize intermediate adsorption energies and suppress detrimental lattice oxygen participation, which often leads to catalyst degradation in harsh environments. While numerous studies have reported enhanced performance through doping strategies, the field has seen a proliferation of empirical listings of materials without sufficient emphasis on the underlying logical connections between dopant selection, structural modifications, and mechanistic improvements. For instance, doping with high-valence REEs such as Er or La can downshift the O 2p band center relative to the Fermi level, increasing the energy barrier for oxygen vacancy formation and thereby mitigating metal dissolution, which is a critical factor in acidic media. This not only stabilizes the catalyst but also fine-tunes the d-band center of TMs, aligning adsorption free energies closer to the Sabatier optimum for OER intermediates. Building on this framework, Hao et al. demonstrated the efficacy of co-doping W and Er into RuO2, where charge redistribution lowers the O 2p band center from -3.31 eV to -4.12 eV, raising the oxygen vacancy formation energy from 0.67 eV to 2.29 eV[124]. This electronic tuning inhibits lattice oxygen oxidation, preventing the formation of soluble Rux>4 species and enabling a low overpotential of 168 mV at 10 mA cm-2 with exceptional stability of 500 h in 0.5 M H2SO4. Furthermore, such mechanisms provide a blueprint for PEMWE applications, where the catalyst sustained 120 h at 100 mA cm-2 without structural collapse, highlighting how electronic modulation translates to practical durability under high-current conditions.
Other rare earth-based catalysts
In addition to La, Ce, and Er, researchers have also explored the use of other REEs to enhance OER performance. Wang et al. developed a series of uniquely doped lanthanide-based nickel-iron layered double hydroxide (NiFe-LDH) nanosheet arrays - namely, NiFeSm-LDH, NiFeCe-LDH, and NiFeLa-LDH - as high-performance electrocatalysts for the OER[125]. Notably, NiFeSm-LDH exhibited outstanding performance, with the lowest overpotential of only 203 mV at a current density of 10 mA cm-2 [Figure 14A]. Structural analyses, in situ Raman spectroscopy, and DFT calculations revealed that the superior catalytic activity arises from a synergistic functionality [Figure 14B and C]. Firstly, due to the lanthanide contraction effect, samarium (Sm) in NiFeSm-LDH can attract more electrons from the outer 3d orbitals of Ni compared to cerium (Ce) in NiFeCe-LDH and lanthanum (La) in NiFeLa-LDH. This leads to a higher Ni valence state and an elevated d-band center. Secondly, in situ Raman spectroscopy shows that the overpotential required to form the NiOOH phase on NiFeSm-LDH is lower (~ 1.32 V) than that on NiFeCe-LDH (1.32-1.37 V) and NiFeLa-LDH (~ 1.37 V), indicating faster reaction kinetics for the Ni2+ to Ni3+ transition in NiFeSm-LDH. Additionally, Wang et al. proposed a sacrificial template-induced nanostructure regulation strategy to fabricate mesoporous perovskite oxide nanosheets (MPONs) with controllable atomic doping [Figure 14D][126]. Among them, Eu-doped LaFeO3 mesoporous perovskite nanosheets exhibited a low overpotential of 267 mV at 10 mA cm-2, outperforming most reported perovskite oxides [Figure 14E]. The excellent catalytic activity of Eu-LaFeO3 MPONs was attributed to their highly porous structure, which increases the density of active sites and enhances lattice oxygen participation, thereby improving intrinsic activity. Wu et al. also found that doping trivalent metals such as gadolinium (Gd), neodymium (Nd), and praseodymium (Pr) into rutile IrO2 significantly enhanced its OER activity under acidic conditions. This enhancement is due to the increased Ir-O covalency[127]. In contrast, doping with higher-valent metals (e.g., +5 or above) weakens the Ir-O covalency and deteriorates OER activity. As shown in the XRD patterns of Figure 14F, A-IrO2-γ exhibits a pure rutile phase. Experimental and theoretical analysis indicates that enhanced Ir-O covalency activates lattice oxygen and facilitates a LOM, thereby accelerating OER kinetics. pH-dependent OER activities were conducted for prepared catalysts, revealing that LOM electrocatalysts exhibit a pH-dependent effect. This is further supported by the linear relationship between the natural logarithm of intrinsic activity and Ir-O covalency described by charge transfer energy. By tuning the Ir-O covalency, the Gd-doped IrO2-σ catalyst achieves an overpotential of only 260 mV at 10 mA cm-2, and demonstrates impressive stability over 200 h in 0.5 M H2SO4 solution [Figure 14G]. Due to their highly tunable electrochemical properties and excellent reactivity, high-entropy materials represent a promising class of next-generation water-splitting catalysts. Zhang et al. successfully prepared a novel high-entropy pyrochlore, (Y0.2Ho0.2Dy0.2Gd0.2Pr0.2)2Ru2O7 (HE-YRO), by equimolar co-doping of REEs - holmium (Ho), dysprosium (Dy), praseodymium (Pr), and gadolinium (Gd) - into the A-site of Y2Ru2O7[128]. As shown in Figure 14H, TEM-EDS mapping confirmed the homogeneous distribution of Ru, Y, Ho, Dy, Gd, and Pr, with atomic ratios of approximately 1: 0.18: 0.22: 0.21: 0.22: 0.23, closely matching the nominal stoichiometry, indicating the formation of a stable high-entropy phase. This catalyst displayed an ultra-low overpotential (η10 = 200 mV) and no observable degradation during stability testing at 10 mA cm-2 [Figure 14I]. Through comparative analysis of pyrochlore oxides, several features contributing to the high OER performance of HE-YRO were identified. First, compared with YRO, HE-YRO contains more low-valent Ru3+ species and oxygen vacancies, which enhance activity. Second, lattice distortion suppresses grain coarsening, resulting in a larger surface area and more accessible active sites for OER. These synergistic effects induced by equimolar multi-element doping at the A-site endow HE-YRO with excellent performance and chemical durability for acidic water oxidation. There are countless reports on rare earth-based OER catalysts. We have listed some representative catalysts and summarized them in Table 2. An intuitive comparative illustration is also presented in Figure 15.
Figure 14. (A) CV curves in O2-saturated 1 M KOH solution at a scan rate of 2 mV/s with 85% iR correction; (B) AEM and (C) LOM pathways for NiFe-LDH, NiFeSm-LDH, NiFeCe-LDH and NiFeLa-LDH. This figure is quoted with permission from Wang et al.[125]; (D) TEM image from the LaFeO3 MPONs; (E) LSV polarization curves of LaFeO3 particles and LaFeO3 MPONs. This figure is quoted with permission from Wang et al.[126]; (F) XRD patterns of A-IrO2-𝛿 (A = Gd, Pr, Nd, Mo, W) and HM-IrO2; (G) OER performance of the prepared electrocatalysts in 0.5 M H2SO4 LSV curves. This figure is quoted with permission from Wu et al.[127]; (H) EDS spectrum of HE-YRO. The signals of Cu and C in the spectrum originated from the copper grid coated by carbon membrane; (I) Polarization curves of HE-YRO and YRO catalysts. This figure is quoted with permission from Zhang et al.[128]. RHE: Reversible hydrogen electrode; CV: cyclic voltammetry; iR: internal resistance; AEM: adsorbate evolution mechanism; LOM: lattice oxygen mechanism; LDH: layered double hydroxide; TEM: transmission electron microscopy; MPON: mesoporous perovskite oxide nanosheet; LSV: linear sweep voltammetry; XRD: X-ray diffraction; OER: oxygen evolution reaction; HM: home-made; EDS: energy-dispersive X-ray spectroscopy. HE-YRO: high-entropy (Y0.2Ho0.2Dy0.2Gd0.2Pr0.2)2Ru2O7.
Figure 15. Comparison of OER electrocatalytic performance across different REE types. TM: transition metal; SLD: surface La-defective; NC: nitrogen-doped carbon; LDH: layered double hydroxide; NF: nickel foam; LSFN: La and S co-doped nickel-iron-based multiphase catalyst; OER: oxygen evolution reaction.
Summary of various REE-based OER electrocatalysts and their OER performance
| Catalysts | REE type | Electrolytes | Overpotential (mV)(η10) | Stability (h) | Ref. |
| NiFeLa | La | 1 M KOH | 201 | 600 | [105] |
| La-CoMoO4 | La | 1 M KOH | 272 (η20) | 45 | [106] |
| La-NiFeLDH /NiS/NF La(CrMnFeCo2Ni) O3HEPO | La La | 1 M KOH 1 M KOH | 190 (iR) 325 | 600 50 | [107] [108] |
| Pt0.1La0.1IrO2@NC | La | 0.5 M H2SO4 | 205 (iR) | 135 | [109] |
| LSFN-63 | La | 1 M KOH | 210 (η100) | 60 | [110] |
| La0.1Ru0.9O2 | La | 0.5 M H2SO4 | 188 | 63 | [111] |
| La3IrO7-SLD | La | 0.1 M HClO4 | 296 (95%iR) | 16 | [112] |
| La-RuO2@TM | La | 0.5 M H2SO4 | 303 (η100) | 120 | [113] |
| CeO2/NiCo2S4 | Ce | 1 M KOH | 146 (90%iR) | 200 | [114] |
| S-Co(OH)2/CeO2 | Ce | 1 M KOH | 227 (95%iR) | 100 | [115] |
| RuO2/CeO2 | Ce | 0.5 M H2SO4 | 180 (90%iR) | 1000 | [116] |
| CeO2/SrIrO3 | Ce | 0.5 M H2SO4 | 238 (85%iR) | 50 | [22] |
| Ce0.21-Co(OH)2 | Ce | 1 M KOH | 300 | 12 | [118] |
| Ce-NiO@MoO2 | Ce | 1 M KOH | 313 (90%iR) | 100 | [119] |
| Ru3MoCeOx | Ce | 0.5 M H2SO4 | 164 (iR) | 100 | [120] |
| NiFeCe-LDH@CP | Ce | 1 M KOH | 232 (iR) | 70 | [121] |
| Er-Co3O4 | Er | 0.5 M H2SO4 | 321 (iR) | 250 | [122] |
| Er-NiFe-LDH@NF | Er | 1 M KOH | 192 | 24 | [123] |
| W0.2Er0.1Ru0.7O2-δ | Er | 0.5 M H2SO4 | 168 (iR) | 500 | [124] |
| NiFeSm-LDH | Sm | 1 M KOH | 203 (85%iR) | 27 | [125] |
| Eu-LaFeO3 | Eu,La | 1 M KOH | 267 | 24 | [126] |
| Gd-IrO2−σ | Gd | 0.5 M H2SO4 | 260 (80%iR) | 200 | [127] |
| Y,Ho,Dy, Gd,Pr | 0.5 M H2SO4 | 200 (iR) | 50 | [128] |
Overall, the electrochemical stability of rare-earth oxides plays a decisive role in determining their practical applicability in OER systems. Pourbaix analysis indicates that La2O3 is stable mainly under alkaline conditions but prone to dissolution in acidic media, whereas CeO2 exhibits superior robustness across a wider pH range owing to its fluorite structure and reversible Ce4+/Ce3+ redox couple that enables oxygen-vacancy formation without bulk degradation. Er2O3 demonstrates improved stability in neutral to alkaline environments due to enhanced lattice binding from lanthanide contraction, yet may still undergo partial oxidation or reconstruction at high anodic potentials. To precisely assess and mitigate such degradation, inductively coupled plasma mass spectrometry (ICP-MS) tracking of dissolved REE and TM species during durability testing is strongly recommended. Moreover, as carbon supports are susceptible to anodic corrosion during OER, the adoption of non-carbon supports (such as TiO2 or conductive oxides) or REE-oxide surface functionalization offers an effective pathway to enhance structural stability and prolong catalyst lifetime.
CONCLUSIONS
REEs, characterized by their unique 4f electronic configurations, multiple accessible oxidation states, and strong affinity for oxygen, exhibit exceptional regulatory potential in both HER and OER. Through precise strategies including doping, defect engineering, alloying, and heterostructure design, these elements enable fine modulation of electronic structures and geometric configurations, optimization of adsorption free energies for reaction intermediates, and reduction of kinetic barriers, collectively enhancing catalytic activity and stability. Beyond conventional architectures, emerging applications of REEs in SACs, HEAs, and multicomponent heterostructures have broken performance limitations of traditional catalysts, paving new avenues for efficient and cost-effective water electrolysis. Recent studies highlight that rare earth-based catalysts can achieve robust bifunctional HER/OER performance across acidic, neutral, and alkaline media, with certain systems surpassing noble-metal benchmarks (e.g., Pt/C and IrO2) in both overpotential and long-term stability. These advances provide a solid theoretical and experimental foundation for understanding the regulatory effect of REEs and guiding rational electrocatalyst design. This review systematically integrates recent advances in rare earth-based electrocatalysis, emphasizing fundamental mechanisms, structure-performance correlations, and emerging design strategies. By evaluating approaches such as doping, defect engineering, alloying, and heterostructure construction across various REEs, it establishes a comprehensive knowledge framework for the research community.
OUTLOOK
The advancement of rare earth-based electrocatalysts from laboratory-scale exploration to large-scale practical application demands a multidisciplinary and sustainability-oriented approach. Future research should first focus on elucidating the dynamic relationship between atomic-scale structure and catalytic performance under realistic operating conditions. The integration of in situ and operando characterization with time-resolved analysis is crucial for identifying active sites, tracking phase transitions, and revealing interfacial reconstruction pathways during HER and OER processes.
Equally important is addressing the sustainability and resource security of REEs. Given the environmental burden and geopolitical constraints associated with REE mining, future catalyst design should emphasize green synthesis, recycling, and closed-loop utilization. Environmentally benign approaches such as solvent-free mechanochemistry, bio-assisted extraction, and low-temperature molten-salt methods can minimize chemical waste and energy consumption. Meanwhile, recycling REEs from spent catalysts, industrial residues, and electronic waste offers a viable route to mitigate supply risks. Advanced recovery technologies including ionic liquid extraction, electrochemical leaching, and membrane-assisted separation are promising for achieving high selectivity and efficiency in REE reclamation. Establishing such circular resource management frameworks will be essential for ensuring the long-term sustainability of REE-based electrocatalytic systems. From a mechanistic perspective, coupling advanced characterization with multiscale theoretical simulations will deepen understanding of how REEs modulate electronic structures, lattice strain, and interfacial charge dynamics. These insights are critical for identifying design principles that bridge the gap between activity, stability, and sustainability. Furthermore, constructing multicomponent hybrid catalysts by integrating REEs with TMs, conductive oxides, or carbon frameworks could generate synergistic effects that enhance bifunctional HER and OER performance and durability. Expanding the exploration beyond the commonly studied La, Ce, and Er to include less-investigated REEs such as Sm, Gd, Dy, Yb, and Lu may uncover new catalytic mechanisms related to 4f electron interactions and spin-polarized charge transfer. In parallel, the application potential of REE-based catalysts in practical electrolyzer systems including PEMWE, AEMWE, and seawater electrolysis requires systematic evaluation. Tailoring REE incorporation strategies to improve corrosion resistance, interfacial stability, and ion transport will be key to achieving long-term operational robustness. The convergence of in situ experimentation, DFT, and machine learning assisted high-throughput screening offers a powerful route to accelerate the rational design and discovery of next-generation REE-based materials.
In summary, rare earth-based electrocatalysts offer a versatile yet resource-sensitive pathway for advancing green hydrogen production. Their future development should not only aim for enhanced catalytic activity and stability but also prioritize environmentally responsible synthesis, efficient recycling, and sustainable resource utilization. By coupling mechanistic insight with circular materials innovation, the field can move toward a more sustainable and scalable future for electrocatalytic water splitting.
DECLARATIONS
Acknowledgments
The authors are grateful for the financial support from the Scientific Research Foundation of the Education Department of Liaoning Province (Grant Nos. LJ212410149030 and LJ212410149009), the National Natural Science Foundation of China (Grant No. 52171121), and the Natural Science Foundation of Inner Mongolia (Grant No. 2024QN02004).
Authors’ contributions
Writing-original draft: Yuan, S.
Writing-review & editing: Wang, Y.; Zhao, H.; Liu, Z. (Zhipeng Liu); Guo, Z.; Li, R.
Collecting & analyzing: Liu, Z. (Zhongyi Liu)
Supervision: Guo, Z.; Li, R.
Funding acquisition: Guo, Z.; Li, R.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorship
This work was supported by the Scientific Research Foundation of the Education Department of Liaoning Province (Grant Nos. LJ212410149030 and LJ212410149009), the National Natural Science Foundation of China (Grant No. 52171121), and the Natural Science Foundation of Inner Mongolia (Grant No. 2024QN02004).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Tian, X.; Zhao, P.; Sheng, W. Hydrogen evolution and oxidation: mechanistic studies and material advances. Adv. Mater. 2019, 31, e1808066.
2. Sun, Y.; Sun, S.; Yang, H.; Xi, S.; Gracia, J.; Xu, Z. J. Spin-related electron transfer and orbital interactions in oxygen electrocatalysis. Adv. Mater. 2020, 32, e2003297.
3. Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390-4.
4. Bin, S.; Chen, Z.; Zhu, Y.; et al. High-pressure proton exchange membrane water electrolysis: current status and challenges in hydrogen production. Int. J. Hydrogen. Energy. 2024, 67, 390-405.
5. Wang, Q.; Lei, Y.; Wang, Y.; et al. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis. Energy. Environ. Sci. 2020, 13, 1593-616.
6. Wang, H. F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-derived electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Soc. Rev. 2020, 49, 1414-48.
7. Wang, C.; Shang, H.; Li, J.; et al. Ultralow Ru doping induced interface engineering in MOF derived ruthenium-cobalt oxide hollow nanobox for efficient water oxidation electrocatalysis. Chem. Eng. J. 2021, 420, 129805.
8. Chen, Z.; Jia, H.; Yuan, J.; et al. N, P-co-doped carbon coupled with CoP as superior electrocatalysts for hydrogen evolution reaction and overall water splitting. Int. J. Hydrogen. Energy. 2019, 44, 24342-52.
9. Hu, C.; Dai, L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019, 31, e1804672.
10. Zhang, A.; Liang, Y.; Zhang, H.; Geng, Z. Zeng, J,. Doping regulation in transition metal compounds for electrocatalysis. Chem. Soc. Rev. 2021, 50, 9817-44.
11. Cho, K. H.; Seo, H.; Park, S.; et al. Uniform, assembled 4nm Mn3O4 nanoparticles as efficient water oxidation electrocatalysts at neutral pH. Adv. Funct. Mater. 2020, 30, 1910424.
12. SMM Metal Market. Rare Earth Oxides Prices. https://www.metal.com/Rare-Earth-Oxides. (accessed 2025-06-02).
13. Gao, W.; Gou, W.; Ma, Y.; Wei, R.; Ho, J. C.; Qu, Y. Cerium phosphate as a novel cocatalyst promoting NiCo2O4 nanowire arrays for efficient and robust electrocatalytic oxygen evolution. ACS. Appl. Energy. Mater. 2019, 2, 5769-76.
14. Lin, X.; Huang, Y. C.; Hu, Z.; et al. 5f covalency synergistically boosting oxygen evolution of UCoO4 catalyst. J. Am. Chem. Soc. 2022, 144, 416-23.
15. Li, M.; Wang, X.; Liu, K.; et al. Ce‐induced differentiated regulation of Co sites via gradient orbital coupling for bifunctional water‐splitting reactions. Adv. Energy. Mater. 2023, 13, 2301162.
16. Wang, X.; Zhu, Y.; Li, H.; Lee, J. M.; Tang, Y.; Fu, G. Rare-earth single-atom catalysts: a new frontier in photo/electrocatalysis. Small. Methods. 2022, 6, e2200413.
17. Rosalbino, F.; Delsante, S.; Borzone, G.; Angelini, E. Electrocatalytic behaviour of Co-Ni-R (R = Rare earth metal) crystalline alloys as electrode materials for hydrogen evolution reaction in alkaline medium. Int. J. Hydrogen. Energy. 2008, 33, 6696-703.
18. Rodney, J. D.; Deepapriya, S.; Das, S. J.; et al. Boosting overall electrochemical water splitting via rare earth doped cupric oxide nanoparticles obtained by co-precipitation technique. J. Alloys. Compd. 2022, 921, 165948.
19. Zhang, F.; Wang, K.; Zhang, H.; et al. Dynamic reconstruction of Ce‐doped Fe2P/NiCoP hybrid for ampere-level oxygen evolution in anion exchange membrane water electrolysis. Adv. Funct. Mater. 2025, 35, 2500861.
20. Ran, B.; Wu, Y.; Zhang, K.; Yao, R.; Li, J.; Liu, G. Supporting IrOx on stable La, Nb-TiO2 with enhanced electron transport for efficient acidic water oxidation. Fuel 2025, 381, 133451.
21. Liu, Y.; Hou, Y.; Cai, M.; et al. Porous yolk@shell-Heterostuctured Co3O4@CeO2/Co3O4 nanospheres as catalysts for the oxygen evolution reaction. ACS. Appl. Nano. Mater. 2024, 7, 21221-30.
22. You, M.; Xu, Y.; He, B.; et al. Realizing robust and efficient acidic oxygen evolution by electronic modulation of 0D/2D CeO2 quantum dots decorated SrIrO3 nanosheets. Appl. Catal. B:. Environ. 2022, 315, 121579.
23. Yan, G.; Wang, Y.; Zhang, Z.; et al. Nanoparticle-decorated ultrathin La2O3 nanosheets as an efficient electrocatalysis for oxygen evolution reactions. Nanomicro. Lett. 2020, 12, 49.
24. Qiu, T.; Tu, B.; Saldana-greco, D.; Rappe, A. M. Ab initio simulation explains the enhancement of catalytic oxygen evolution on CaMnO3. ACS. Catal. 2018, 8, 2218-24.
25. Li, M.; Wang, Y.; Zheng, Y.; et al. Gadolinium-induced valence structure engineering for enhanced oxygen electrocatalysis. Adv. Energy. Mater. 2020, 10, 1903833.
26. Li, C.; Wang, P.; He, M.; Yuan, X.; Fang, Z.; Li, Z. Rare earth-based nanomaterials in electrocatalysis. Coord. Chem. Rev. 2023, 489, 215204.
27. Wan, Y.; Wei, W.; Li, L.; Wu, L.; Qin, H.; Yuan, X. Modulating support effect in high-entropy sulfide via La single-atom for boosted oxygen evolution. Small 2025, 21, e2502039.
28. Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-noble metal-based carbon composites in hydrogen evolution reaction: fundamentals to applications. Adv. Mater. 2017, 29.
29. Mahmood, N.; Yao, Y.; Zhang, J. W.; Pan, L.; Zhang, X.; Zou, J. J. Electrocatalysts for hydrogen evolution in alkaline electrolytes: mechanisms, challenges, and prospective solutions. Adv. Sci. (Weinh). 2018, 5, 1700464.
30. Dehkordi, H. B.; Zhiani, M. A novel Ir-Ru-based nanoparticle supported on ordered electrochemically synthesized TiO2-nanotube as a highly active and stable oxygen evolution reaction catalyst for water splitting in acidic media. Int. J. Hydrogen. Energy. 2023, 48, 33042-61.
31. Huang, C.; Zhou, J.; Duan, D.; et al. Roles of heteroatoms in electrocatalysts for alkaline water splitting: a review focusing on the reaction mechanism. Chin. J. Catal. 2022, 43, 2091-110.
32. Jiang, Y.; Fu, H.; Liang, Z.; Zhang, Q.; Du, Y. Rare earth oxide based electrocatalysts: synthesis, properties and applications. Chem. Soc. Rev. 2024, 53, 714-63.
34. Mclemore, V. Rare earth elements (REE) deposits associated with great plain margin deposits (alkaline-related), Southwestern United States and Eastern Mexico. Resources 2018, 7, 8.
35. Tao, Y.; Shen, L.; Feng, C.; et al. Distribution of rare earth elements (REEs) and their roles in plant growth: a review. Environ. Pollut. 2022, 298, 118540.
36. Fu, H.; Jiang, Y.; Zhang, M.; et al. High-entropy rare earth materials: synthesis, application and outlook. Chem. Soc. Rev. 2024, 53, 2211-47.
37. Ganduglia-pirovano, M. V.; Hofmann, A.; Sauer, J. Oxygen vacancies in transition metal and rare earth oxides: current state of understanding and remaining challenges. Surf. Sci. Rep. 2007, 62, 219-70.
38. Strasser, P.; Koh, S.; Anniyev, T.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nature. Chem. 2010, 2, 454-60.
39. Cao, Y.; Zheng, X.; Deng, Y.; Hu, W. Comprehensive insight into electronic modulation of rare-earth elements for enhancing electrocatalytic performance of atomically dispersed materials. Adv. Funct. Mater. 2025, 35, 2423158.
40. Wang, X.; Li, M.; Tang, Y.; Li, H.; Fu, G. Rare earths evoked gradient orbital coupling in electrocatalysis: recent advances and future perspectives. Prog. Mater. Sci. 2026, 155, 101539.
41. Liu, T.; Chen, Y.; Wang, X.; et al. Rare-earth oxychlorides as promoters of ruthenium toward high-performance hydrogen evolution electrocatalysts for alkaline electrolyzers. Adv. Mater. 2025, 37, e2417621.
42. Kaup, G.; Meiners, D.; Kynast, U. H. Rare earth Ibuprofen complexes: Highlighting a pharmaceutical. Opt. Mater. 2017, 63, 26-31.
43. Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285-303.
44. Zhou, B.; Li, Z.; Chen, C. Global potential of rare earth resources and rare earth demand from clean technologies. Minerals 2017, 7, 203.
45. Royal Society of Chemistry Periodic Table. https://www.rsc.org/periodic-table. (accessed 2025-06-02).
46. Fan, C.; Dong, W.; Saira, Y.; Tang, Y.; Fu, G.; Lee, J. M. Rare-earth-modified metal-organic frameworks and derivatives for photo/electrocatalysis. Small 2023, 19, e2302738.
47. Yin, L.; Zhang, S.; Huang, Y.; Yan, C.; Du, Y. Cerium contained advanced materials: shining star under electrocatalysis. Coord. Chem. Rev. 2024, 518, 216111.
48. Nosaka, Y.; Nosaka, A. Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302-36.
49. Zhang, J.; Chen, J.; Luo, Y.; et al. Engineering heterointerfaces coupled with oxygen vacancies in lanthanum-based hollow microspheres for synergistically enhanced oxygen electrocatalysis. J. Energy. Chem. 2021, 60, 503-11.
50. Fu, G.; Li, W.; Zhang, J. Y.; et al. Facilitating the deprotonation of OH to O through Fe4+ -induced states in perovskite LaNiO3 enables a fast oxygen evolution reaction. Small 2021, 17, e2006930.
51. Gao, W.; Ma, F.; Wang, C.; Wen, D. Ce dopant significantly promotes the catalytic activity of Ni foam-supported Ni3S2 electrocatalyst for alkaline oxygen evolution reaction. J. Power. Sources. 2020, 450, 227654.
52. Shen, Z.; Wei, X.; Wang, M.; et al. Multi-defect Pd-based catalyst doped with rare earth element La for ethanol-assisted energy-saving hydrogen production. J. Colloid. Interface. Sci. 2025, 697, 137969.
53. Munawar, T.; Bashir, A.; Nisa, M. U.; et al. Unravelling the operando structural and chemical stability of rare earth metals co-doped CeO2-based electrocatalysts for oxygen evolution reaction. Int. J. Hydrogen. Energy. 2025, 137, 1097-106.
54. Zhu, M.; Gao, J.; Zhang, C. La-doping-induced lattice strain and electronic state modulation in RuO2 for electrocatalytic oxygen evolution in acidic solutions. Inorg. Chem. 2025, 64, 4571-9.
55. Yang, J.; Shen, Y.; Xian, J.; Xiang, R.; Li, G. Rare-earth element doped NiFe-MOFs as efficient and robust bifunctional electrocatalysts for both alkaline freshwater and seawater splitting. Chem. Sci. 2025, 16, 685-92.
56. Yang, J.; An, L.; Wang, S.; et al. Defects engineering of layered double hydroxide-based electrocatalyst for water splitting. Chin. J. Catal. 2023, 55, 116-36.
57. Yan, D.; Xia, C.; Zhang, W.; et al. Cation defect engineering of transition metal electrocatalysts for oxygen evolution reaction. Adv. Energy. Mater. 2022, 12, 2202317.
58. Yan, X.; Jia, Y.; Yao, X. Defective structures in metal compounds for energy-related electrocatalysis. Small. Struct. 2020, 2, 2000067.
59. Wang, Z.; Xu, W.; Chen, X.; et al. Defect-rich nitrogen doped Co3O4/C porous nanocubes enable high-efficiency bifunctional oxygen electrocatalysis. Adv. Funct. Mater. 2019, 29, 1902875.
60. Huang, Q.; Xia, G. J.; Huang, B.; et al. Activating lattice oxygen by a defect-engineered Fe2O3-CeO2 nano-heterojunction for efficient electrochemical water oxidation. Energy. Environ. Sci. 2024, 17, 5260-72.
61. Wang, Z.; Li, T.; Wang, Q. Plasma-engineered CeOx nanosheet array with nitrogen-doping and porous architecture for efficient electrocatalysis. Nanomaterials. 2024, 14, 185.
62. Zou, Y.; Rukundo, E.; Chen, X.; Rao, X.; Liu, Y. Enhanced formic acid electrosynthesis from carbon dioxide by mediating the Bi2O3/La2O3 interface and La-induced oxygen vacancies in BiLa MOF-derived nanocatalysts. Sep. Purif. Technol. 2025, 353, 128624.
63. Jiang, Y.; Liang, Z.; Fu, H.; Hai, G.; Du, Y. Utilizing oxygen vacancies in cerium oxide to narrow the gap between d and f band centers for efficient alkaline water oxidation. Nano. Today. 2025, 62, 102705.
64. Zhang, Y.; Nie, K.; Li, B.; et al. Cerium-optimized platinum-free high-entropy alloy nanoclusters for enhanced ampere-level sustainable hydrogen generation. Appl. Catal. B:. Environ. Energy. 2025, 360, 124529.
65. Li, Y.; Yuan, X.; Wang, P.; et al. Rare earth alloy nanomaterials in electrocatalysis. J. Energy. Chem. 2023, 83, 574-94.
66. Zhang, Y. Review of the structural, magnetic and magnetocaloric properties in ternary rare earth RE2T2X type intermetallic compounds. J. Alloys. Compd. 2019, 787, 1173-86.
67. Zhang, S.; Yin, L.; Du, Y. Regulating Rh-based rare earth nanoalloy electrocatalysts by scandium, yttrium for accelerated hydrogen evolution kinetics. J. Rare. Earths. 2025, 43, 1188-94.
68. Zhang, S.; Yin, L.; Wang, S.; et al. Ternary rare earth alloy Pt3-xIrxSc nanoparticles modulate negatively charged Pt via charge transfer to facilitate pH-universal hydrogen evolution. ACS. Nano. 2023, 17, 23103-14.
69. Xia, J.; Zhao, H.; Huang, B.; et al. Efficient optimization of electron/oxygen pathway by constructing ceria/hydroxide interface for highly active oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1908367.
70. Zhao, G.; Rui, K.; Dou, S. X.; Sun, W. Boosting electrochemical water oxidation: the merits of heterostructured electrocatalysts. J. Mater. Chem. A. 2020, 8, 6393-405.
71. Wu, W.; Luo, S.; Huang, Y.; He, H.; Shen, P. K.; Zhu, J. Recent advances in transition metal phosphide-based heterostructure electrocatalysts for the oxygen evolution reaction. Mater. Chem. Front. 2024, 8, 1064-83.
72. Song, X. Z.; Su, Q. F.; Li, S. J.; et al. Heterostructural Co/CeO2/Co2P/CoP@NC dodecahedrons derived from CeO2-inserted zeolitic imidazolate framework-67as efficient bifunctional electrocatalysts for overall water splitting. Int. J. Hydrogen. Energy. 2020, 45, 30559-70.
73. Chen, J.; Hu, Z.; Ou, Y.; et al. Interfacial engineering regulated by CeOx to boost efficiently alkaline overall water splitting and acidic hydrogen evolution reaction. J. Mater. Sci. Technol. 2022, 120, 129-38.
74. Munawar, T.; Bashir, A.; Nadeem, M. S.; et al. Core-shell CeO2@C60 hybrid serves as a dual-functional catalyst: photocatalyst for organic pollutant degradation and electrocatalyst for oxygen evolution reaction. Ceram. Int. 2023, 49, 8447-62.
75. Flores-Melo, L. M.; Arce-Estrada, E.; Trujillo-Olivares, I.; Sandoval-Pineda, J. M.; Reyes-Rodríguez, J. L.; González-Huerta, R. d. G. Influence of CeO2 nanoparticles in the stability of electrodeposited Ni anodes for alkaline electrolysers. Int. J. Hydrogen. Energy. 2023, 48, 18141-53.
76. Li, F.; Jiang, M.; Lai, C.; Xu, H.; Zhang, K.; Jin, Z. Yttrium- and cerium-codoped ultrathin metal-organic framework nanosheet arrays for high-efficiency electrocatalytic overall water splitting. Nano. Lett. 2022, 22, 7238-45.
77. Ma, Y.; Mu, G.; Miao, Y.; et al. Hydrangea flower‐like nanostructure of dysprosium‐doped Fe‐MOF for highly efficient oxygen evolution reaction. Rare. Metals. 2021, 41, 844-50.
78. Wei, P.; Li, X.; He, Z.; et al. Electron density modulation of MoP by rare earth metal as highly efficient electrocatalysts for pH-universal hydrogen evolution reaction. Appl. Catal. B:. Environ. 2021, 299, 120657.
79. Ye, X.; Ma, H.; Wu, S.; et al. Electron structure customization of molybdenum phosphide via lanthanum doping toward highly efficient overall water splitting. J. Mater. Sci. Technol. 2025, 218, 227-35.
80. Wu, Y.; Liu, Y.; Liu, K.; et al. Hierarchical and self-supporting honeycomb LaNi5 alloy on nickel foam for overall water splitting in alkaline media. Green. Energy. Environ. 2022, 7, 799-806.
81. Li, W.; Sun, Z.; Ge, R.; et al. Nanoarchitectonics of La‐doped Ni3S2/MoS2 hetetostructural electrocatalysts for water electrolysis. Small. Struct. 2023, 4, 2300175.
82. Jin, Y.; Fan, X.; Li, Q.; et al. Self-reconstruction of high entropy alloys for efficient alkaline hydrogen evolution. Small 2025, 21, e2408165.
83. Wang, Z. L.; Huang, G. Y.; Zhu, G. R.; et al. La-exacerbated lattice distortion of high entropy alloys for enhanced electrocatalytic water splitting. Appl. Catal. B:. Environ. Energy. 2025, 361, 124585.
84. Wu, Y.; Yao, R.; Zhao, Q.; Li, J.; Liu, G. La-RuO2 nanocrystals with efficient electrocatalytic activity for overall water splitting in acidic media: synergistic effect of La doping and oxygen vacancy. Chem. Eng. J. 2022, 439, 135699.
85. Rodney, J. D.; Deepapriya, S.; Cyril Robinson, M.; et al. Lanthanum doped copper oxide nanoparticles enabled proficient bi-functional electrocatalyst for overall water splitting. Int. J. Hydrogen. Energy. 2020, 45, 24684-96.
86. Guo, X.; Li, M.; Qiu, L.; et al. Engineering electron redistribution of bimetallic phosphates with CeO2 enables high-performance overall water splitting. Chem. Eng. J. 2023, 453, 139796.
87. Ding, X.; Yu, J.; Huang, W.; Chen, D.; Lin, W.; Xie, Z. Modulation of the interfacial charge density on Fe2P-CoP by coupling CeO2 for accelerating alkaline electrocatalytic hydrogen evolution reaction and overall water splitting. Chem. Eng. J. 2023, 451, 138550.
88. Guo, Q.; Li, Y.; Xu, Z.; Liu, R. CeO2‐accelerated surface reconstruction of CoSe2 nanoneedle forms active CeO2@CoOOH interface to boost oxygen evolution reaction for water splitting. Adv. Energy. Mater. 2024, 15, 2403744.
89. Liu, X.; Wei, S.; Cao, S.; et al. Lattice strain with stabilized oxygen vacancies boosts ceria for robust alkaline hydrogen evolution outperforming benchmark Pt. Adv. Mater. 2024, 36, e2405970.
90. Liu, K.; Liu, T.; Wu, X.; et al. Frustrated Lewis pair chemistry in 2D CeO2 for efficient alkaline hydrogen evolution. J. Mater. Chem. A. 2024, 12, 28843-52.
91. Gao, W.; Yan, M.; Cheung, H. Y.; et al. Modulating electronic structure of CoP electrocatalysts towards enhanced hydrogen evolution by Ce chemical doping in both acidic and basic media. Nano. Energy. 2017, 38, 290-6.
92. Zhang, M.; Xu, H.; Yang, H.; et al. Electronic modulation of nickel cobalt phosphide nanosheets by Ce doping for efficient overall water splitting. Small 2025, 21, e2504837.
93. Li, N.; Zhang, L.; Zhang, H.; et al. Synergistic effect between Er‐doped MoS2 nanosheets and interfacial Mo-N coupling phases for enhanced electrocatalytic hydrogen evolution. Rare. Metals. 2023, 43, 1301-8.
94. Zhang, G.; Wang, B.; Bi, J.; Fang, D.; Yang, S. Constructing ultrathin CoP nanomeshes by Er-doping for highly efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A. 2019, 7, 5769-78.
95. Zhang, H.; Aierke, A.; Zhou, Y.; et al. A high‐performance transition‐metal phosphide electrocatalyst for converting solar energy into hydrogen at 19.6% STH efficiency. Carbon. Energy. 2022, 5, e217.
96. Nadeem, M.; Yasin, G.; Bhatti, M. H.; Mehmood, M.; Arif, M.; Dai, L. Pt-M bimetallic nanoparticles (M = Ni, Cu, Er) supported on metal organic framework-derived N-doped nanostructured carbon for hydrogen evolution and oxygen evolution reaction. J. Power. Sources. 2018, 402, 34-42.
97. Chen, G.; Chen, W.; Lu, R.; et al. Near-atomic-scale superfine alloy clusters for ultrastable acidic hydrogen electrocatalysis. J. Am. Chem. Soc. 2023, 145, 22069-78.
98. Fatima, S.; Sajid, I. H.; Khan, M. F.; Rizwan, S. Synthesis and characterization of erbium decorated V2CTx for water splitting properties. Int. J. Hydrogen. Energy. 2024, 55, 110-7.
99. Xu, X.; Chen, Y.; Zhou, W.; et al. A perovskite electrocatalyst for efficient hydrogen evolution reaction. Adv. Mater. 2016, 28, 6442-8.
100. Liu, W.; Tan, W.; He, H.; Peng, Y.; Chen, Y.; Yang, Y. One-step electrodeposition of Ni-Ce-Pr-Ho/NF as an efficient electrocatalyst for hydrogen evolution reaction in alkaline medium. Energy 2022, 250, 123831.
101. Xu, Y.; Zhou, Q.; Ren, T.; et al. Enhanced electron penetration triggering interfacial charge redistribution in N-doped graphene-wrapped NiGd nanoparticles for coupling methanol electroreforming to H2 production. J. Mater. Chem. A. 2023, 11, 20112-9.
102. Liu, J.; Chen, Y.; Shi, Y.; et al. Asymmetrical Nd-O-Ru bridge active sites boost hydrogen evolution at large current densities. Int. J. Hydrogen. Energy. 2025, 175, 151493.
103. Hu, C.; Zhang, L.; Gong, J. Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy. Environ. Sci. 2019, 12, 2620-45.
104. Jiang, H.; Gu, J.; Zheng, X.; et al. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy. Environ. Sci. 2019, 12, 322-33.
105. Wang, X.; Pi, W.; Hu, S.; Bao, H.; Yao, N.; Luo, W. Boosting oxygen evolution reaction performance on NiFe-based catalysts through d-orbital hybridization. Nanomicro. Lett. 2024, 17, 11.
106. Arumugam, B.; Siddharthan, E. E.; Mannu, P.; et al. Regulating the electronic structure of CoMoO4 via La doping for efficient and durable electrochemical water splitting reactions. J. Mater. Chem. A. 2025, 13, 6749-67.
107. Song, Q.; Li, K.; Cao, Z.; et al. Doping La into NiFe LDH/NiS heterostructure achieving high-current-density oxygen evolution for anion exchange membrane water electrolysis. Chem. Eng. J. 2025, 504, 157526.
108. Nguyen, T. X.; Liao, Y.; Lin, C.; Su, Y.; Ting, J. Advanced high entropy perovskite oxide electrocatalyst for oxygen evolution reaction. Adv. Funct. Mater. 2021, 31, 2101632.
109. Hao, S.; Wang, Y.; Zheng, G.; et al. Tuning electronic correlations of ultra-small IrO2 nanoparticles with La and Pt for enhanced oxygen evolution performance and long-durable stability in acidic media. Appl. Catal. B:. Environ. 2020, 266, 118643.
110. Gan, Y.; Ye, Y.; Dai, X.; et al. La and S Co-doping induced the synergism of multiphase nickel-iron nanosheets with rich oxygen vacancies to trigger large-current-density oxygen evolution and urea oxidation reactions. Small 2023, 19, e2303250.
111. Li, C. H.; Yuan, C. Z.; Huang, X.; et al. Tailoring the electron redistribution of RuO2 by constructing a Ru-O-La asymmetric configuration for efficient acidic oxygen evolution. eScience 2025, 5, 100307.
112. Qin, Q.; Jang, H.; Wang, Y.; et al. Gettering La Effect from La3IrO7 as a highly efficient electrocatalyst for oxygen evolution reaction in acid media. Adv. Energy. Mater. 2020, 11, 2003561.
113. Zhang, X. Y.; Yin, H.; Dang, C. C.; et al. Unlocking enhanced catalysis stability in acidic oxygen evolution: structural insights for PEM applications under high-current density. Angew. Chem. Int. Ed. Engl. 2025, 64, e202425569.
114. Wang, P.; Han, X.; Bai, P.; et al. Utilizing an electron redistribution strategy to inhibit the leaching of sulfur from CeO2/NiCo2S4 heterostructure for high-efficiency oxygen evolution. Appl. Catal. B:. Environ. Energy. 2024, 344, 123659.
115. Zhi, Y.; Li, Z.; Tang, Y.; et al. “Electron-reservoir” CeO2 layer on S-Co(OH)2 to stabilize lattice oxygen for boosting oxygen evolution reaction at large current density. Nano. Energy. 2025, 134, 110565.
116. Song, H.; Yong, X.; Waterhouse, G. I.; et al. RuO2-CeO2 lattice matching strategy enables robust water oxidation electrocatalysis in acidic media via two distinct oxygen evolution mechanisms. ACS. Catal. 2024, 14, 3298-307.
117. Liu, M.; Min, K.; Han, B.; Lee, L. Y. S. Interfacing or doping? Role of Ce in Highly promoted water oxidation of NiFe‐layered double hydroxide. Adv. Energy. Mater. 2021, 11, 2101281.
118. Zhou, Y. N.; Fan, R. Y.; Dou, S. Y.; et al. Tailoring electron transfer with Ce integration in ultrathin Co(OH)2 nanosheets by fast microwave for oxygen evolution reaction. J. Energy. Chem. 2021, 59, 299-305.
119. He, H.; Shang, F.; An, B.; et al. Hierarchical core-shell Ce-doped NiO@MoO2 architecture with Ni 3d-band center modulation for enhanced high-current-density oxygen evolution. Appl. Catal. B:. Environ. Energy. 2024, 358, 124455.
120. He, J.; Li, W.; Xu, P.; Sun, J. Tuning electron correlations of RuO2 by co-doping of Mo and Ce for boosting electrocatalytic water oxidation in acidic media. Appl. Catal. B:. Environ. 2021, 298, 120528.
121. Liao, Y.; He, R.; Pan, W.; et al. Lattice distortion induced Ce-doped NiFe-LDH for efficient oxygen evolution. Chem. Eng. J. 2023, 464, 142669.
122. Pan, S.; Li, H.; Wang, T.; et al. Erratum: Addition to “Er-doping enhances the oxygen evolution performance of cobalt oxide in acidic medium”. ACS. Catal. 2025, 15, 7516-7.
123. Zhu, Y.; Wang, X.; Zhu, X.; et al. Improving the oxygen evolution activity of layered double-hydroxide via erbium-induced electronic engineering. Small 2023, 19, e2206531.
124. Hao, S.; Liu, M.; Pan, J.; et al. Dopants fixation of Ruthenium for boosting acidic oxygen evolution stability and activity. Nat. Commun. 2020, 11, 5368.
125. Wang, M.; Chen, K.; Yan, Z.; Chen, Y.; Liu, H.; Du, X. Tailoring the oxygen evolution reaction activity of lanthanide-doped NiFe-LDHs through lanthanide contraction. Chem. Eng. J. 2024, 496, 154059.
126. Wang, B.; Wu, X.; Jia, S.; et al. Ultrahigh specific surface area mesoporous perovskite oxide nanosheets with rare-earth-enhanced lattice oxygen participation for superior water oxidation. J.Mater. Science. Technol. 2025, 227, 255-61.
127. Wu, J.; Zou, W.; Zhang, J.; et al. Regulating Ir-O covalency to boost acidic oxygen evolution reaction. Small 2024, 20, e2308419.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data



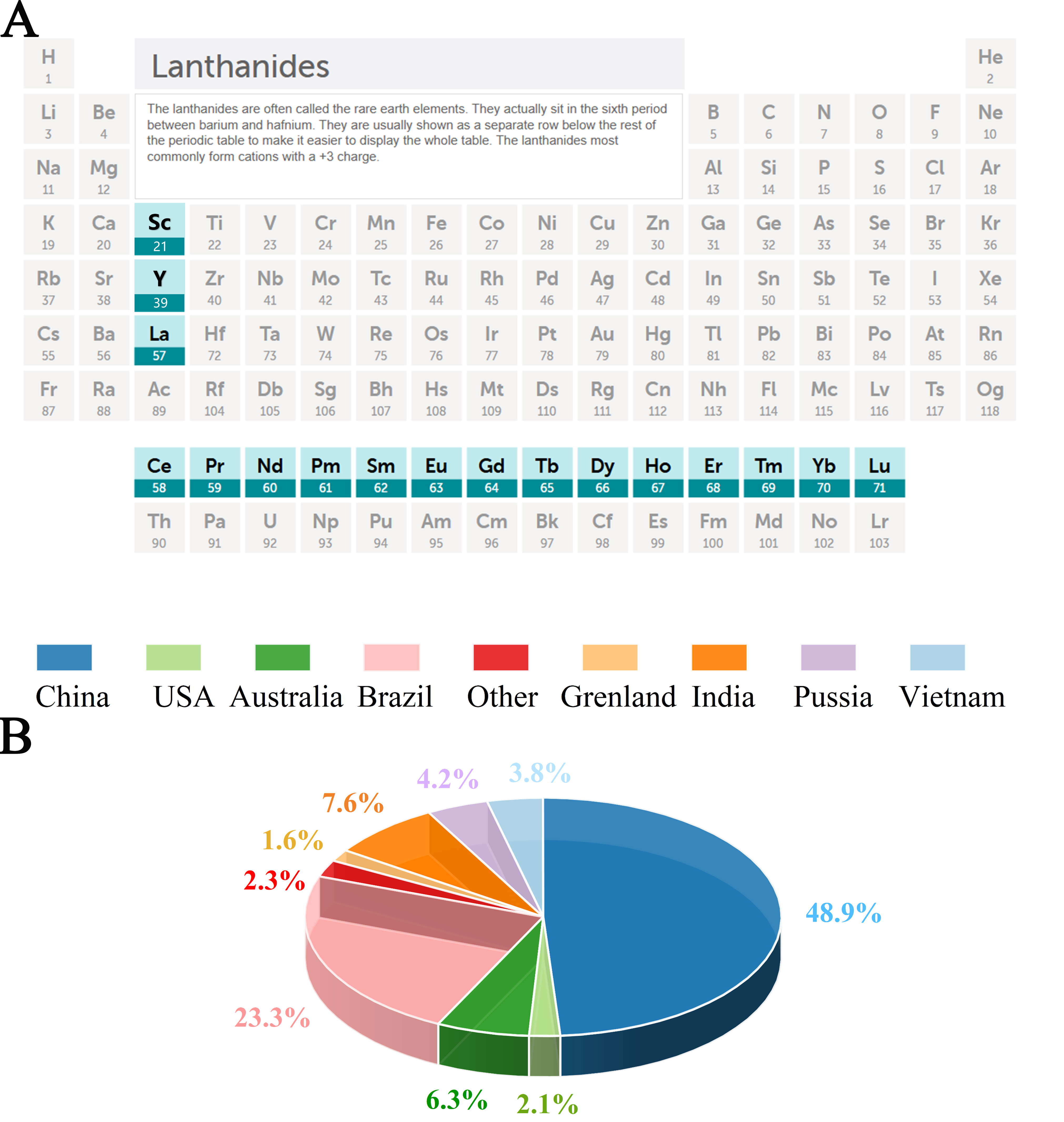
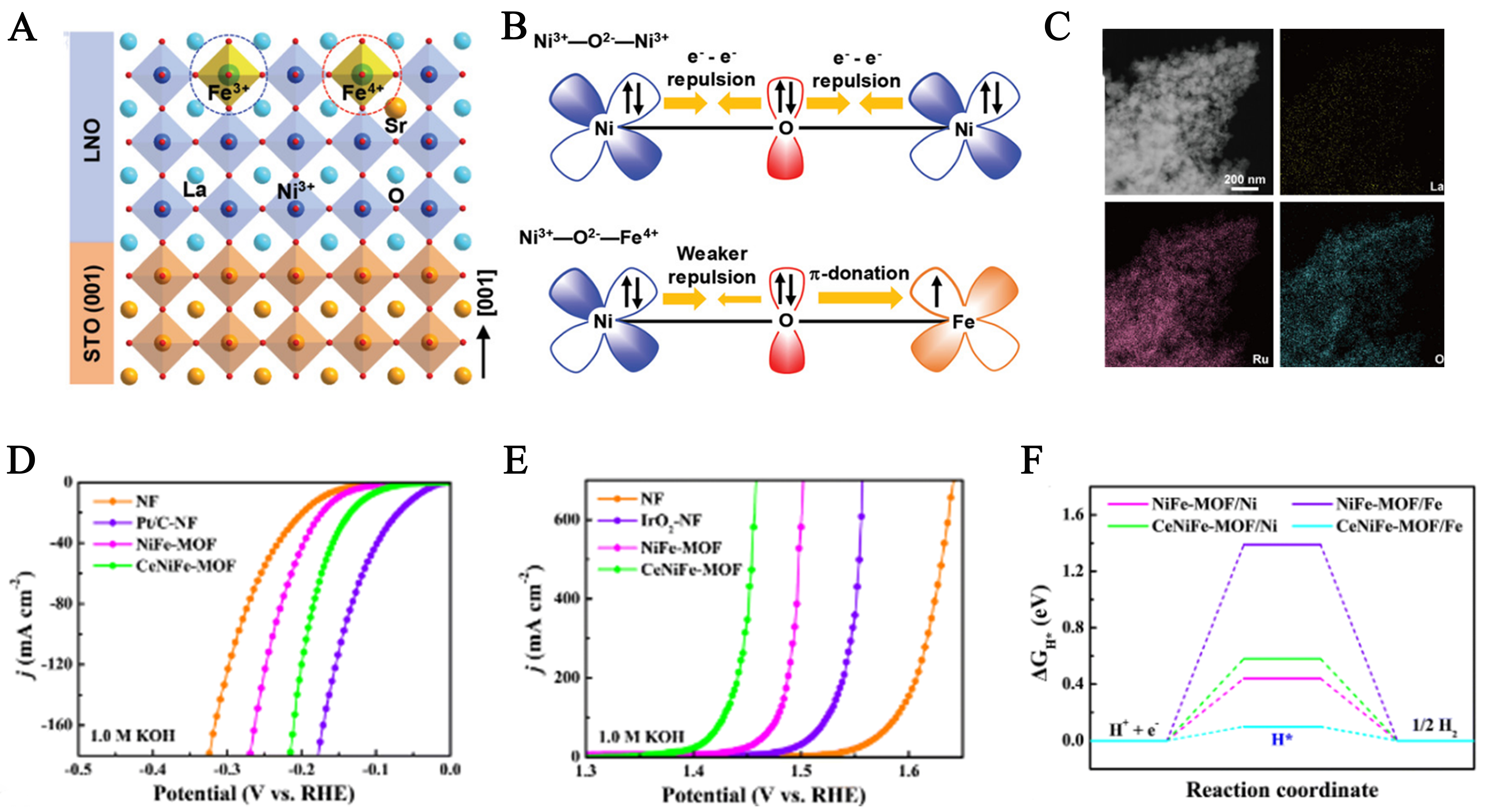
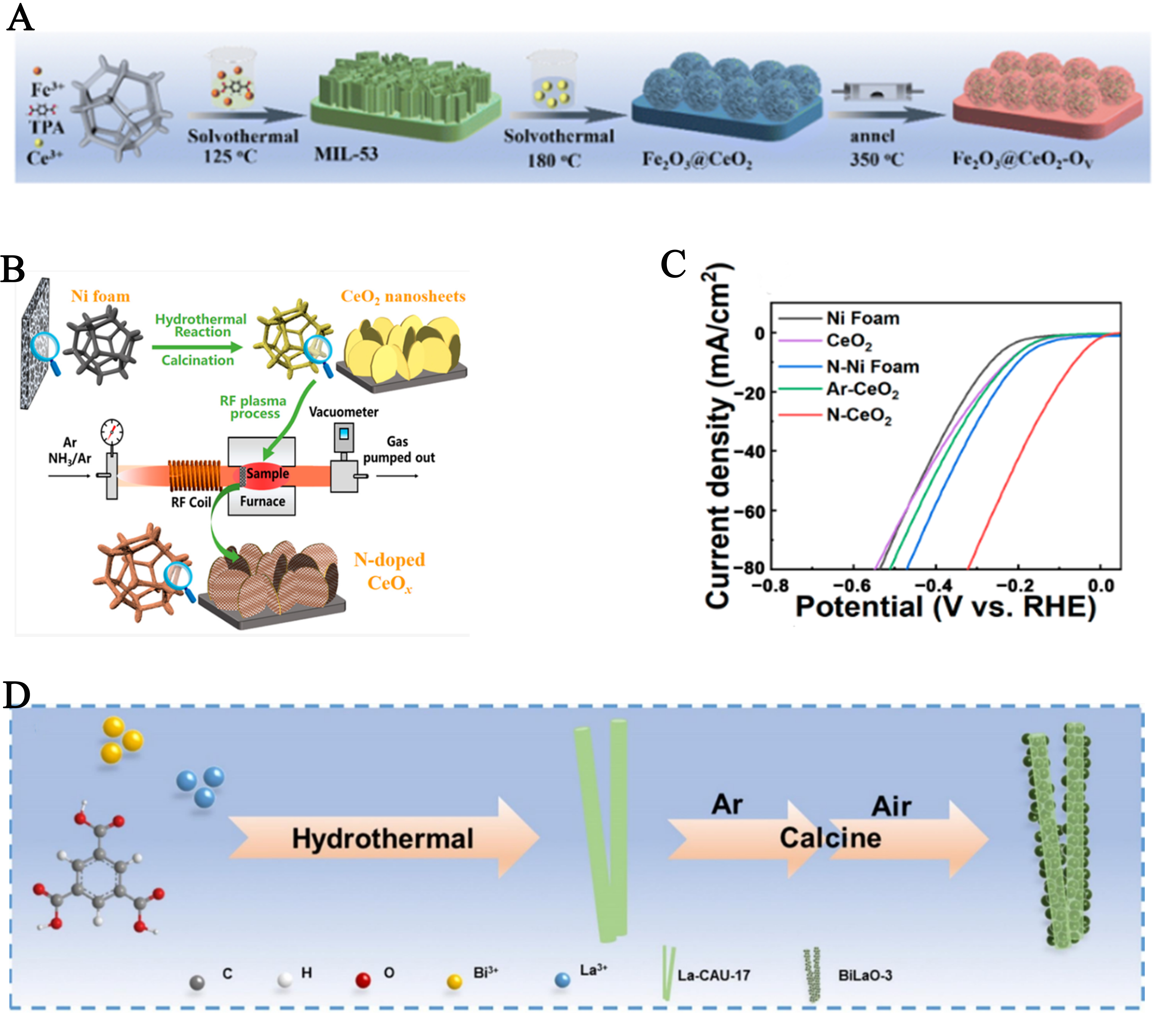
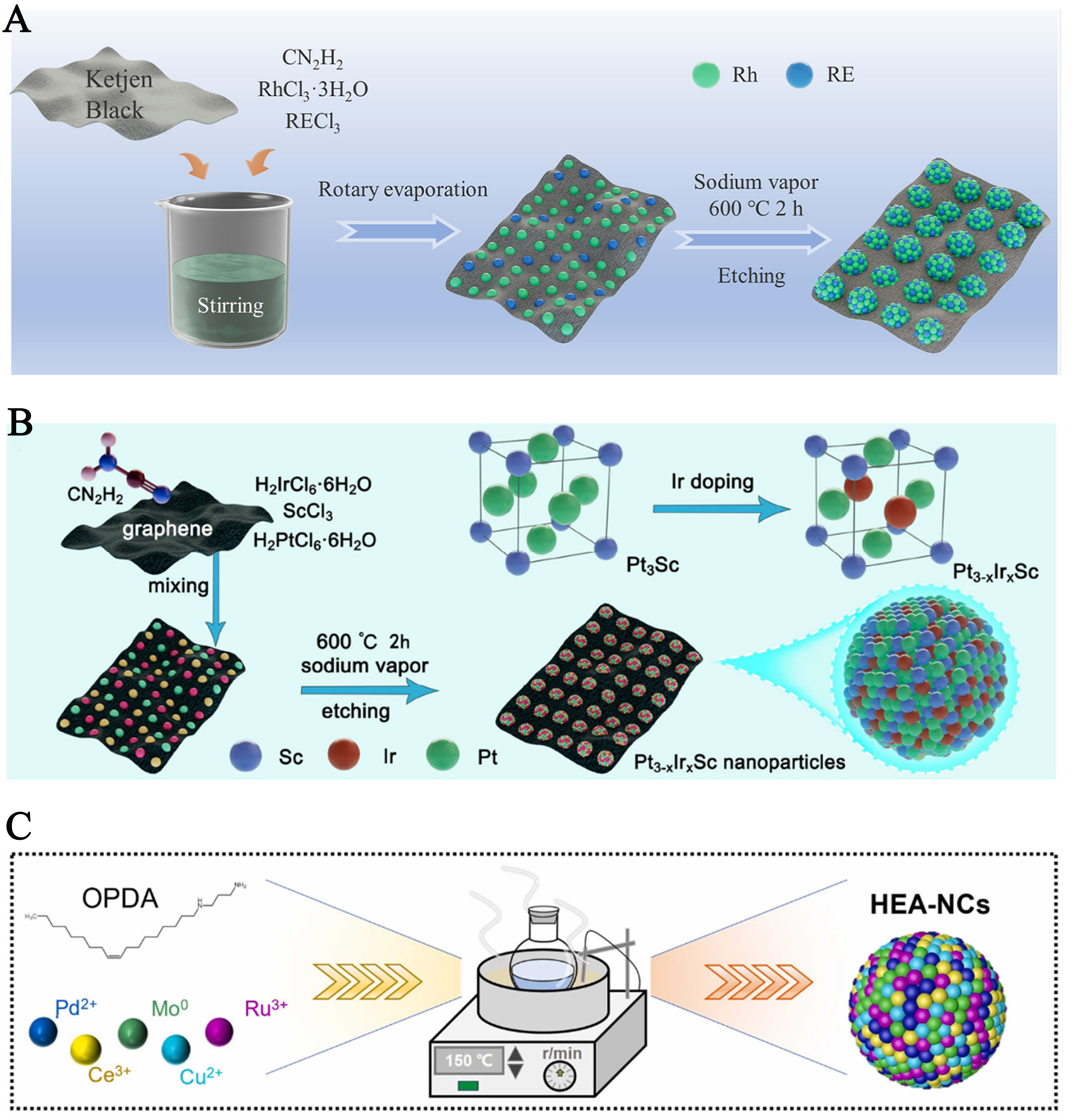
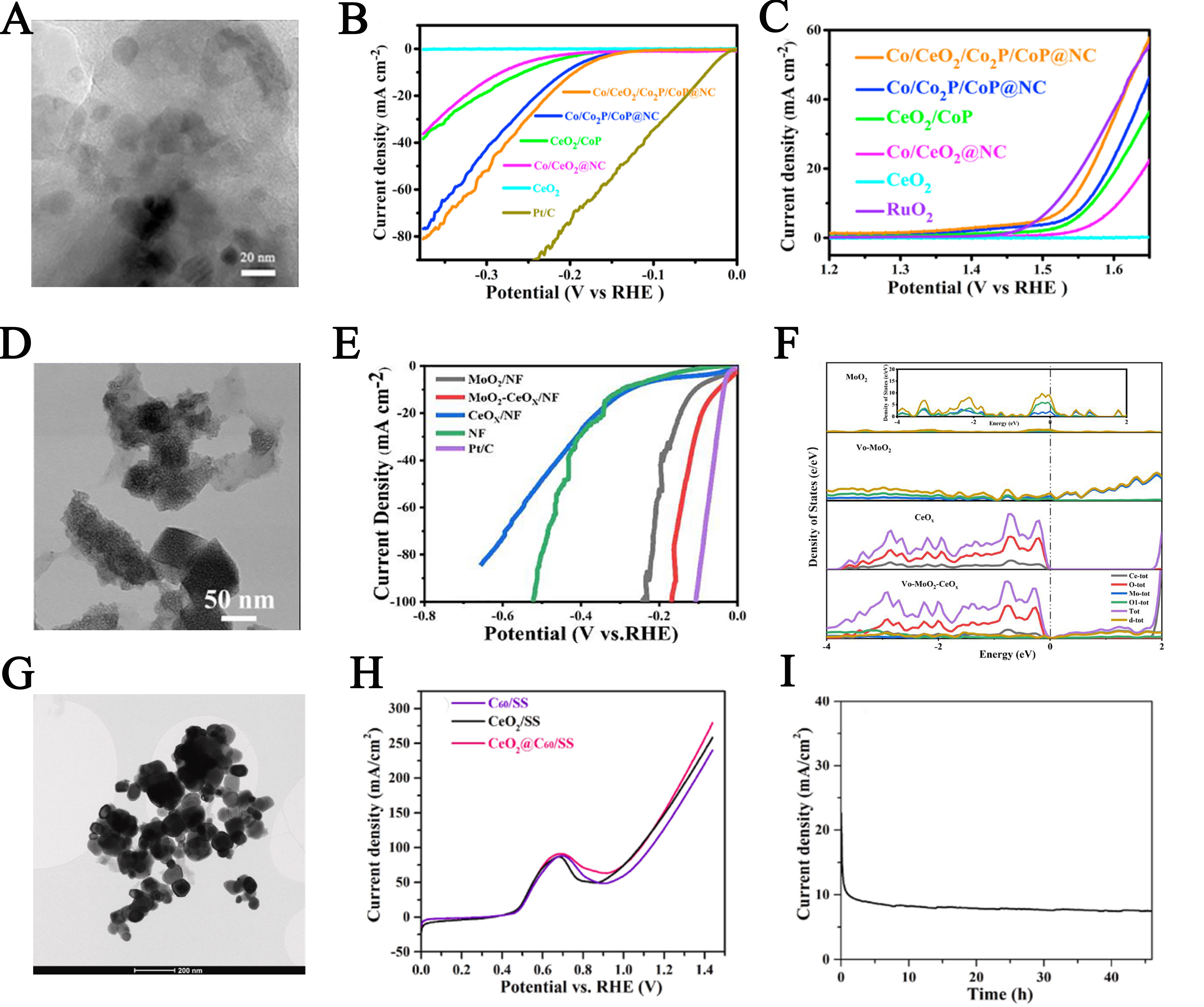
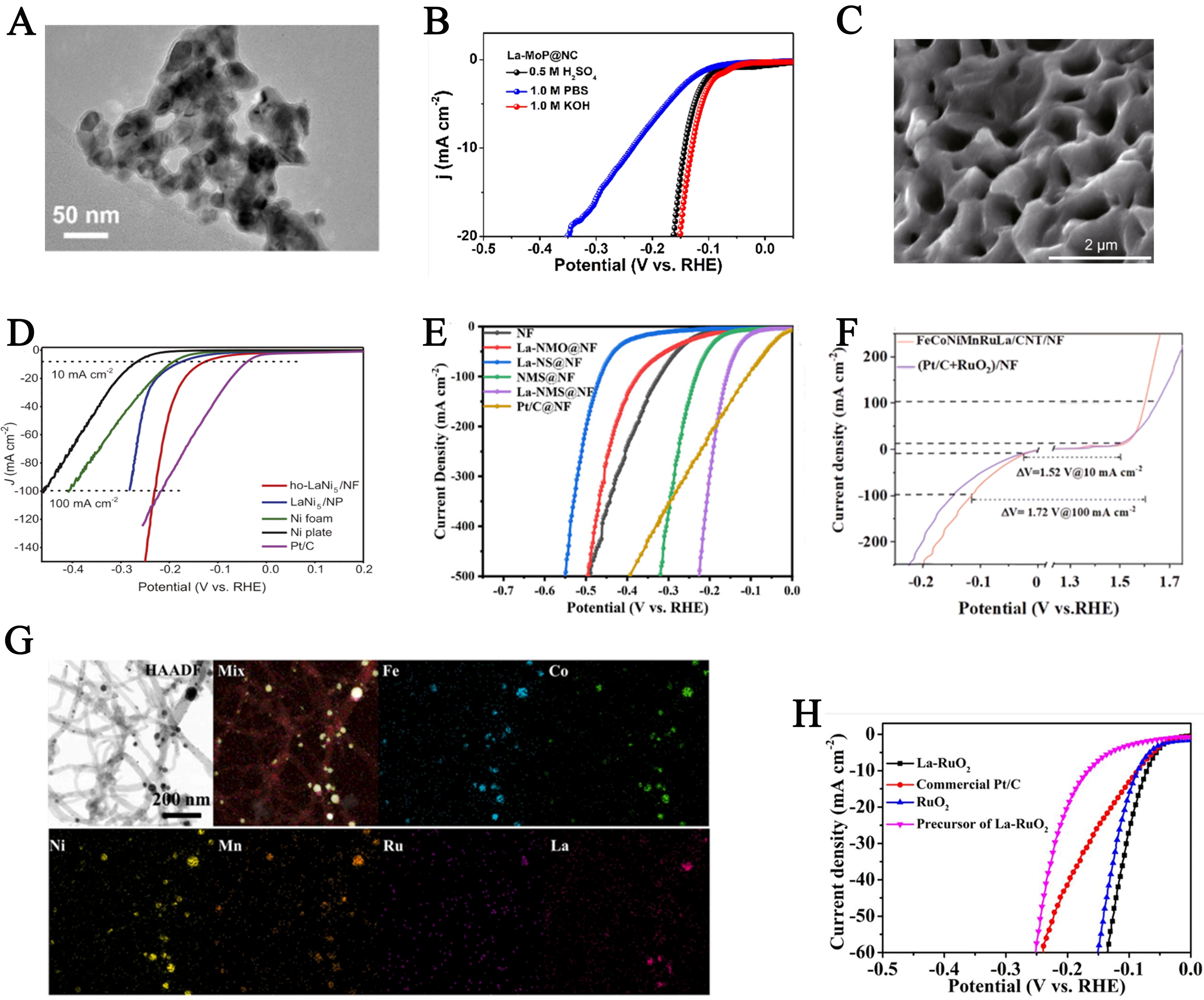
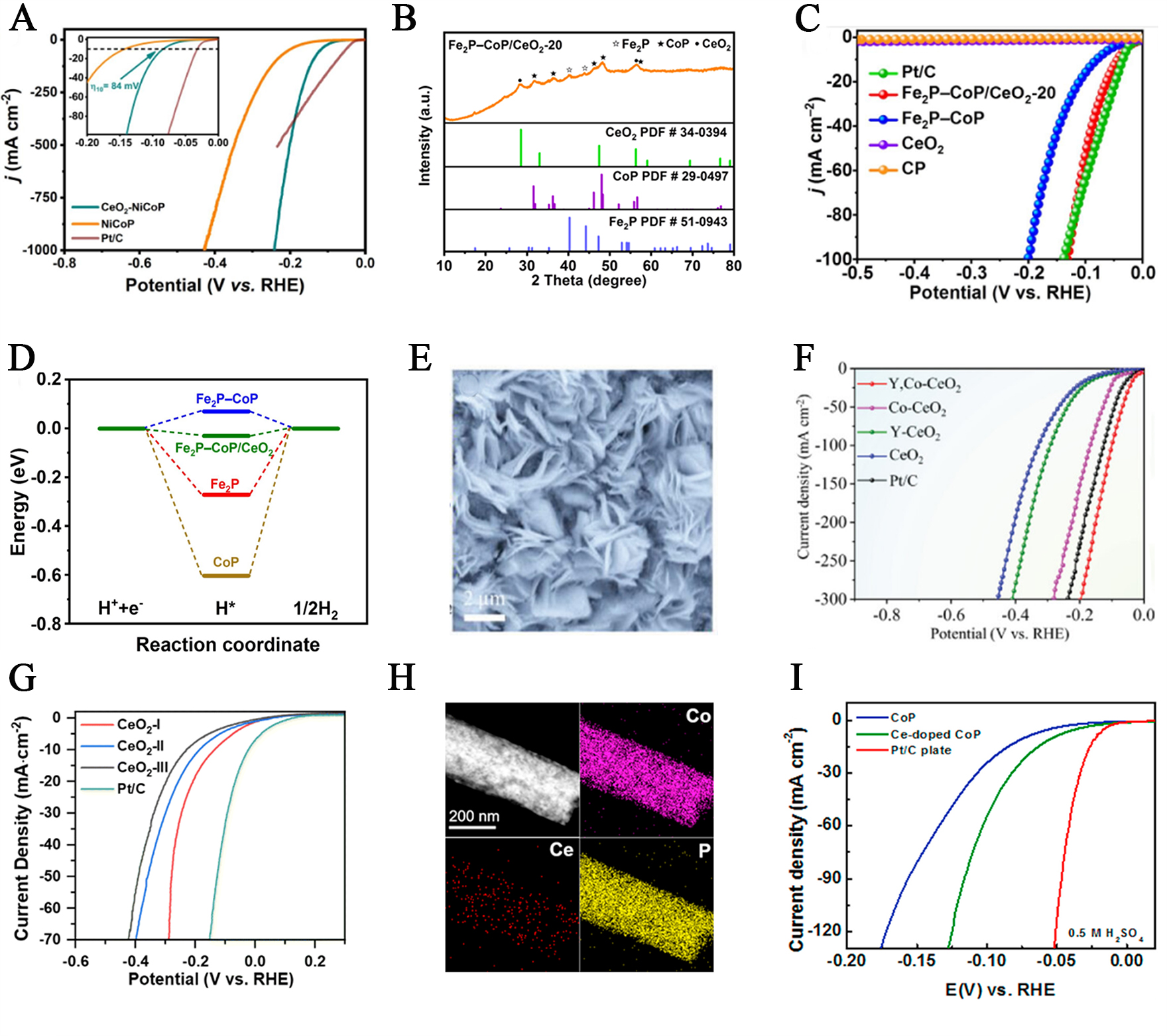
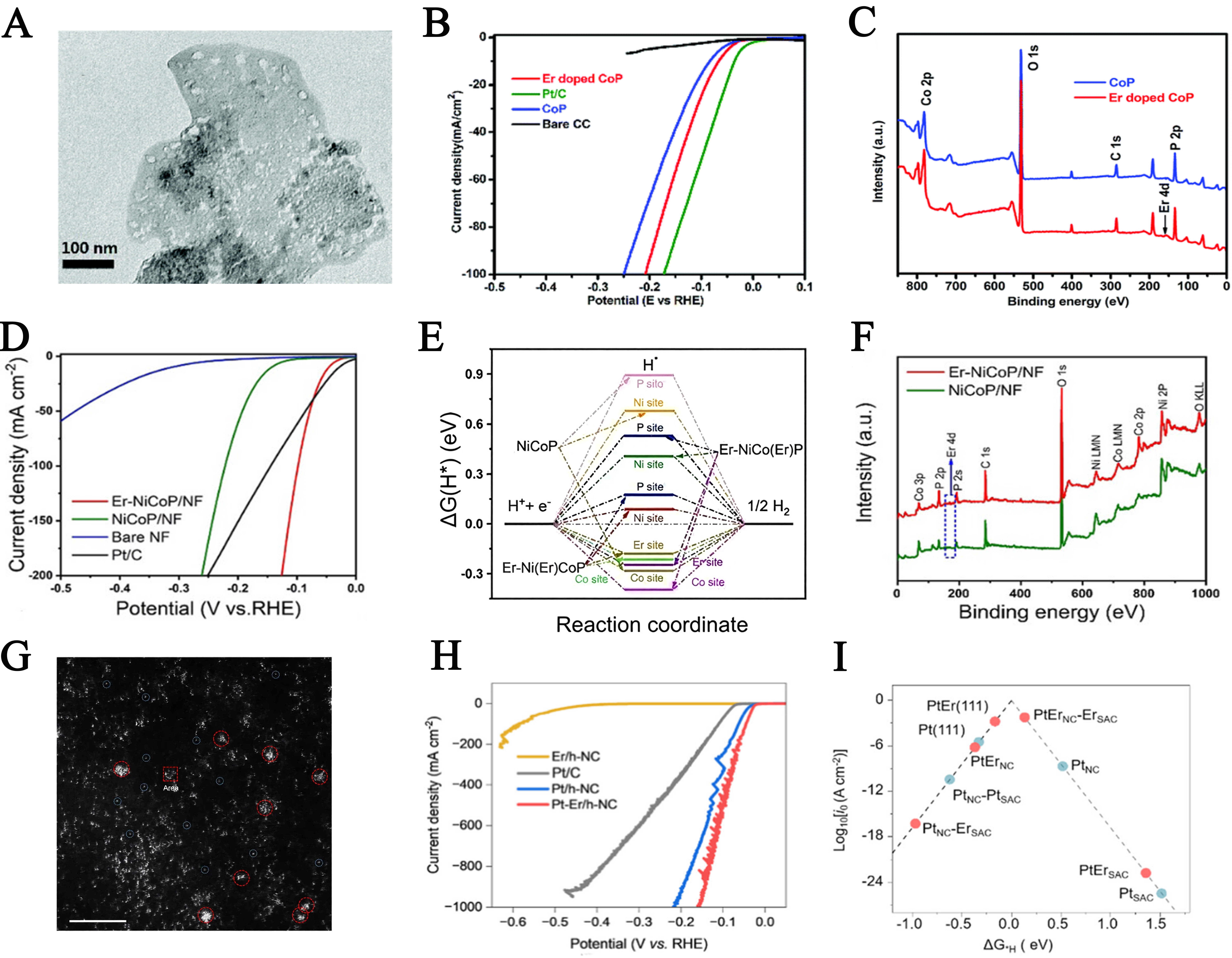
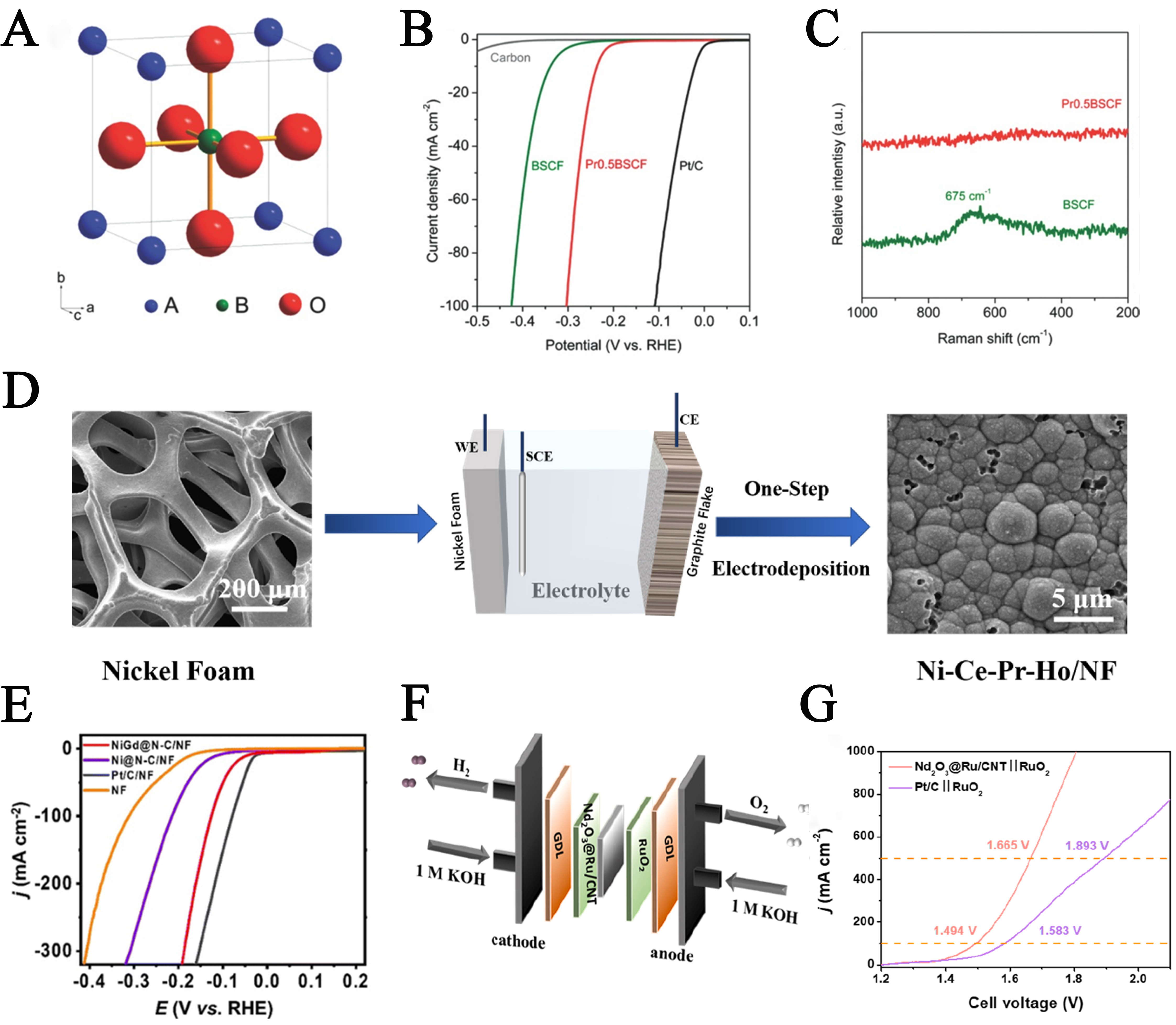
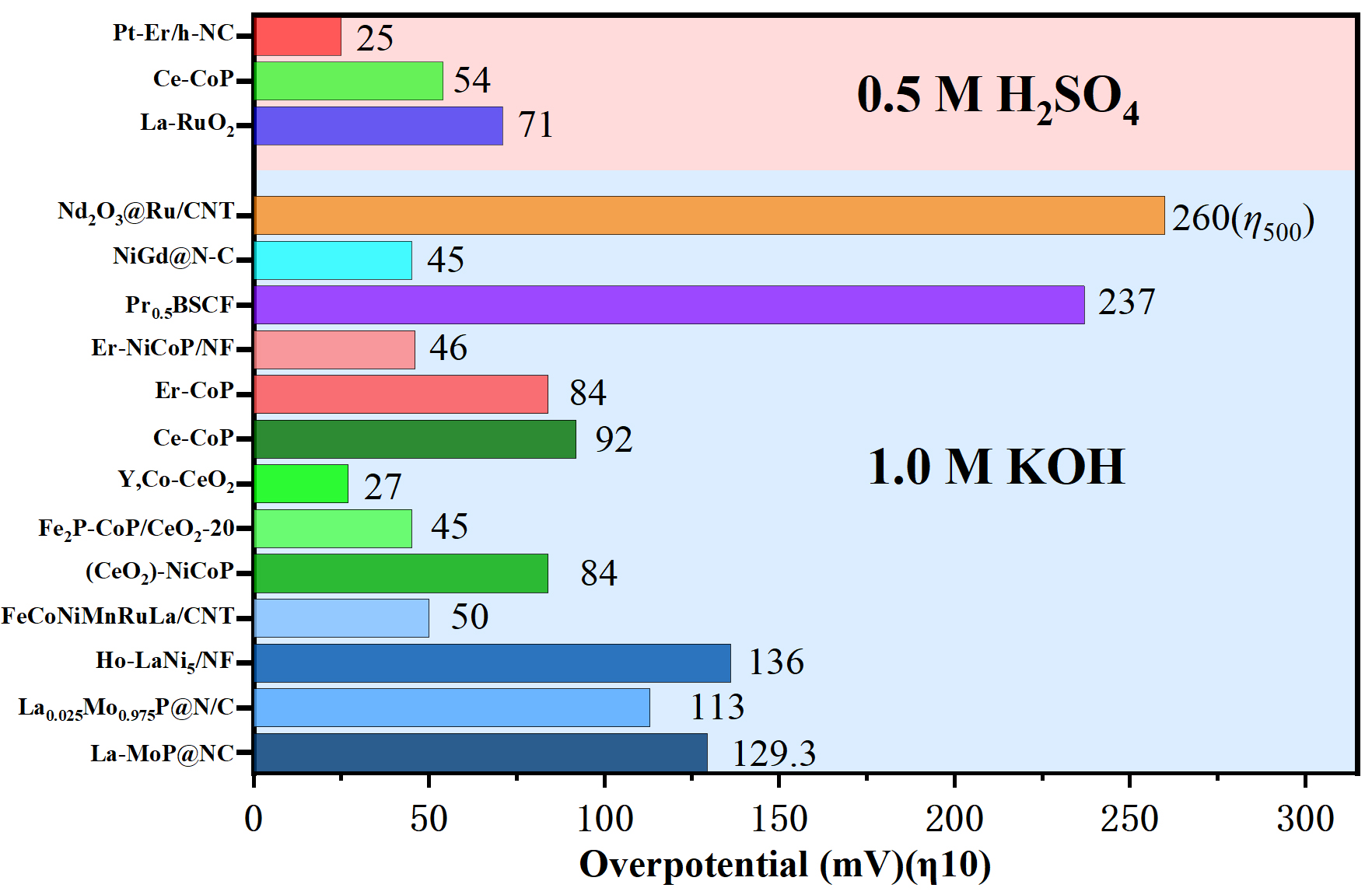
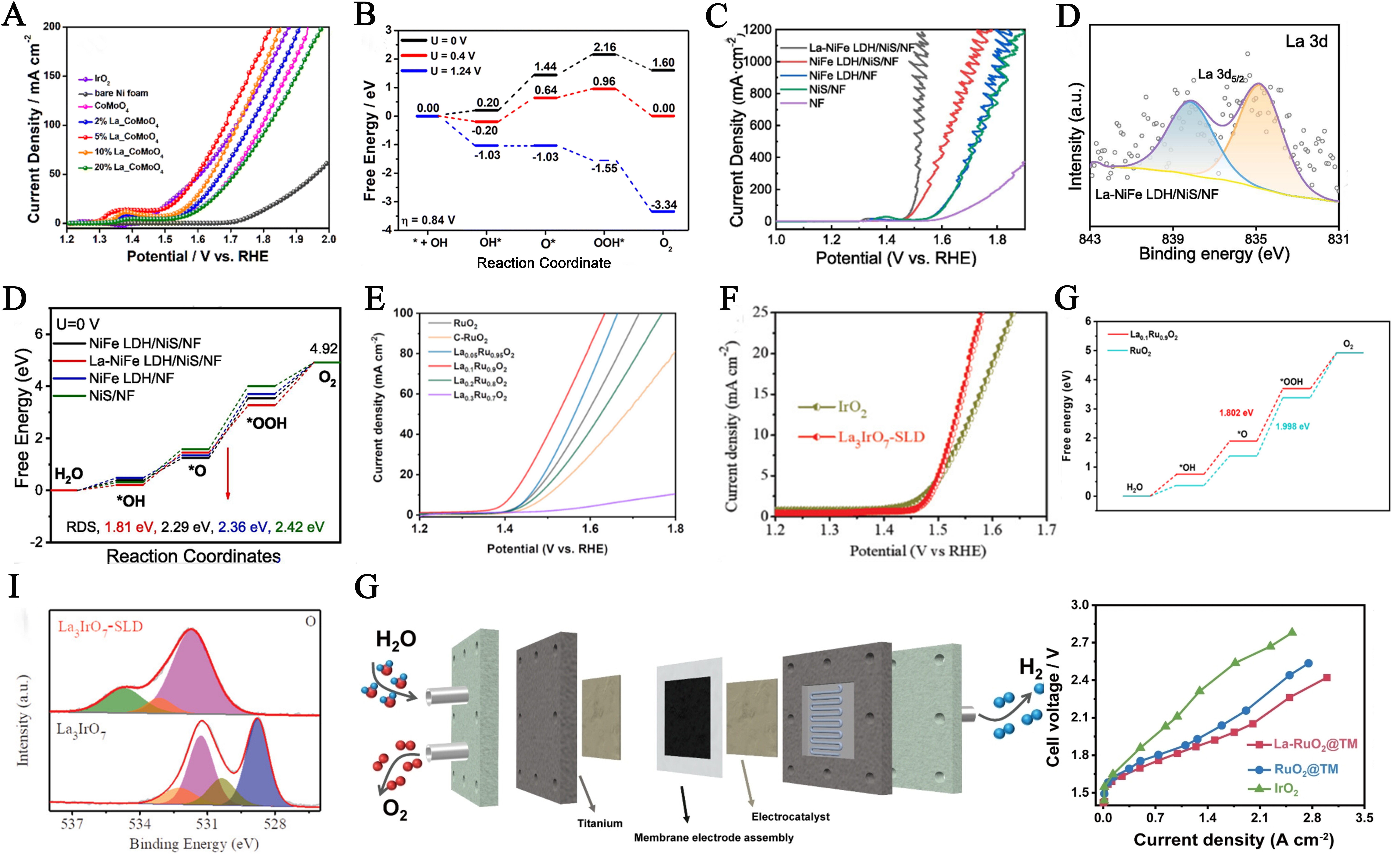
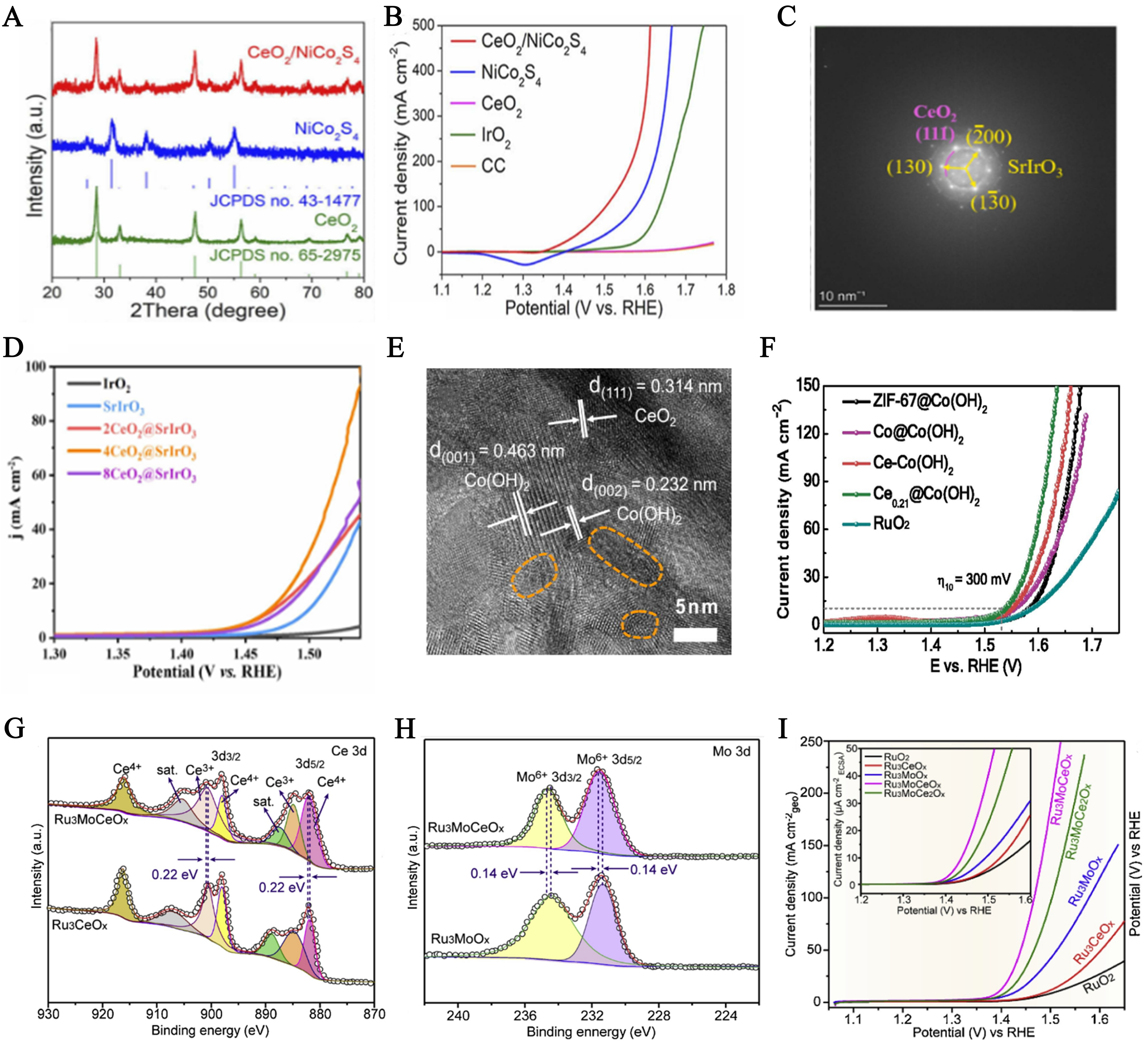
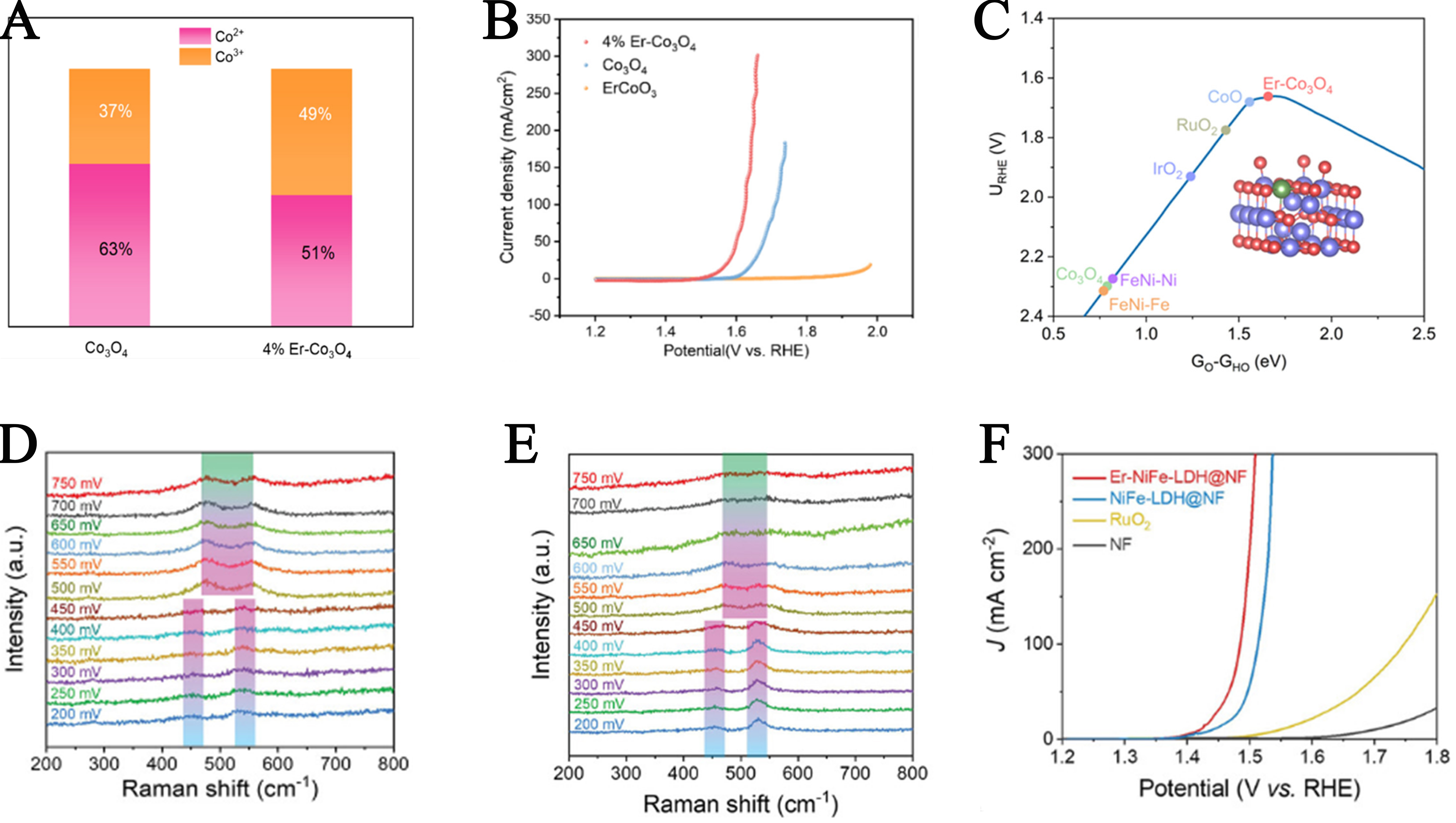
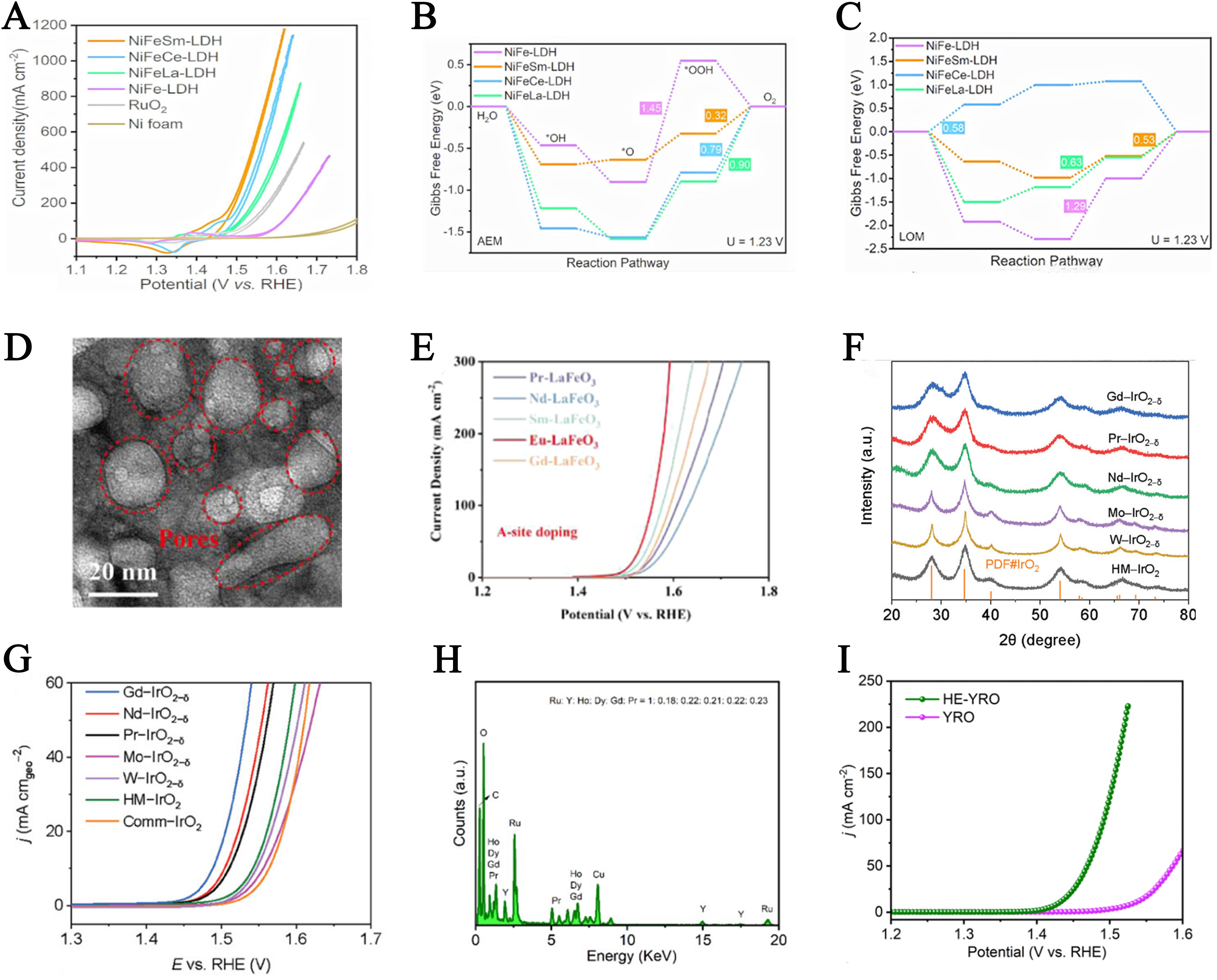
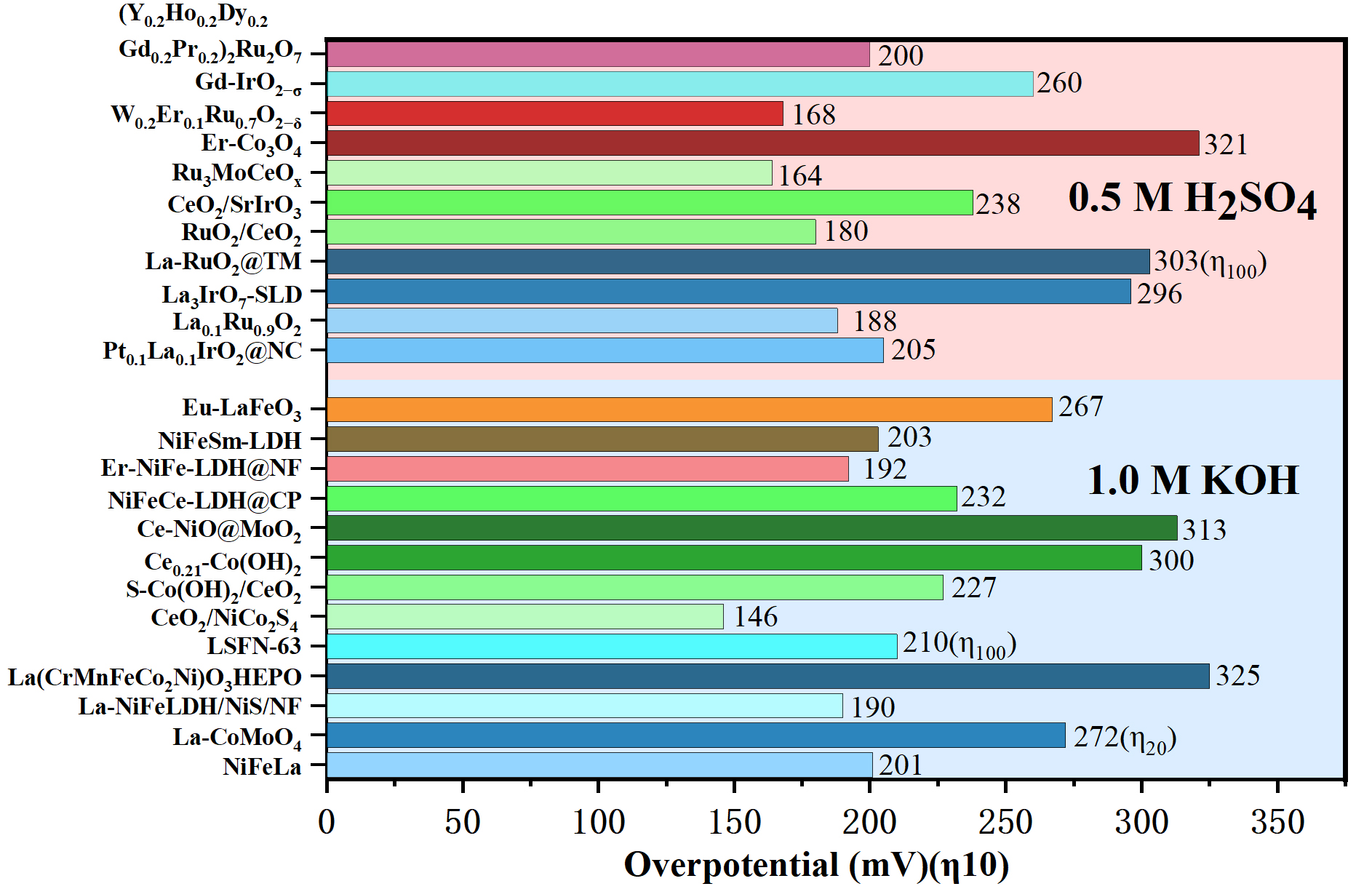








Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at [email protected].